Rachel A. Powsner - Essentials of Nuclear Medicine Physics, Instrumentation, and Radiation Biology
Здесь есть возможность читать онлайн «Rachel A. Powsner - Essentials of Nuclear Medicine Physics, Instrumentation, and Radiation Biology» — ознакомительный отрывок электронной книги совершенно бесплатно, а после прочтения отрывка купить полную версию. В некоторых случаях можно слушать аудио, скачать через торрент в формате fb2 и присутствует краткое содержание. Жанр: unrecognised, на английском языке. Описание произведения, (предисловие) а так же отзывы посетителей доступны на портале библиотеки ЛибКат.
- Название:Essentials of Nuclear Medicine Physics, Instrumentation, and Radiation Biology
- Автор:
- Жанр:
- Год:неизвестен
- ISBN:нет данных
- Рейтинг книги:4 / 5. Голосов: 1
-
Избранное:Добавить в избранное
- Отзывы:
-
Ваша оценка:
- 80
- 1
- 2
- 3
- 4
- 5
Essentials of Nuclear Medicine Physics, Instrumentation, and Radiation Biology: краткое содержание, описание и аннотация
Предлагаем к чтению аннотацию, описание, краткое содержание или предисловие (зависит от того, что написал сам автор книги «Essentials of Nuclear Medicine Physics, Instrumentation, and Radiation Biology»). Если вы не нашли необходимую информацию о книге — напишите в комментариях, мы постараемся отыскать её.
Essentials of Nuclear Medicine Physics, Instrumentation, and Radiation Biology — читать онлайн ознакомительный отрывок
Ниже представлен текст книги, разбитый по страницам. Система сохранения места последней прочитанной страницы, позволяет с удобством читать онлайн бесплатно книгу «Essentials of Nuclear Medicine Physics, Instrumentation, and Radiation Biology», без необходимости каждый раз заново искать на чём Вы остановились. Поставьте закладку, и сможете в любой момент перейти на страницу, на которой закончили чтение.
Интервал:
Закладка:
6 You receive a dose of 99mTc measuring 370 MBq from the radiopharmacy at 10 am. Your patient does not arrive in the department until 2 pm. How much activity, in mCi, remains? (The T1/2 of 99mTc is 6 hours. The constant e = 2.718).
7 Rank the following binding energies from greatest to least:Electron binding energy for outer shell electrons.Nuclear binding energy.Electron binding energy for inner shell electrons.
8 True or false: The term metastable refers to an intermediate state of nuclear decay lasting longer than 10–12 seconds prior to undergoing isomeric transition.
9 Which of the following is true regarding beta decay of a specific radioisotope:The energy of the emitted beta particle is always the same.The energy of the emitted antineutrino is always the same.The summed energy of the emitted beta particle and antineutrino is always the same.
10 Which unit of measurement for radioactivity is defined as one radioactive decay per second?Bequerel.Millicurie.Megabequerel.
11 10 mCi equals how many MBq?2.7 MBq.37 MBq.270 MBq.370 MBq.
12 Lighter nuclides (Z < 83) with an excess of neutrons tend to decay by:Gamma emission.Beta minus decay.Isomeric transition.Positron emission.Alpha emission.
13 Which of the following statements are true?An alpha particle is the same thing as a helium nucleus.Neutrinos have the same charge as an electron.X‐rays always have lower energies than gamma rays.The terms “activity” and “count rate” are the same—they express a measurement of photons per second.
14 When orbital electrons move from an outer shell to an inner‐shell, which of the following is not trueCharacteristic X‐rays can be emitted.Auger electrons can be emitted.The atom becomes more stable.A mixture of gamma rays and internal conversion electrons can be emitted.
Answers
1 (c)
2 (1) d. (2) None of the above; usually used as a geological term. (3) None of the above; in nuclear medicine it refers to an element whose nucleus is in an unstable (excited) state. (4) (c). (5) (a).
3 (b) and (c) are true. (a) is false; technetium does not have a stable form; 99Tc has a T1/2 of 2.1 × 105 year.
4 (e).
5 (d).
6 6.3 mCi.
7 Nuclear binding energy, electron binding energy for inner shell electrons, electron binding energy for outer shell electrons.
8 True.
9 (3).
10 Bequerel.
11 370 MBq.
12 (2) Beta minus decay.
13 (1) only.
14 (d).
CHAPTER 2 Interaction of Radiation with Matter
When radiation strikes matter, both the nature of the radiation and the composition of the matter affect what happens. The process begins with the transfer of radiation energy to the atoms and molecules, heating the matter or even modifying its structure.
If all the energy of a bombarding particle or photon is transferred, the radiation will appear to have been stopped within the irradiated matter. Conversely, if the energy is not completely deposited in the matter, the remaining energy will emerge as though the matter were transparent or at least translucent. This said, we will now introduce some of the physical phenomena involved as radiation interacts with matter, and in particular we shall consider, separately at first, the interactions in matter of both photons (gamma rays and X‐rays) and charged particles (alpha and beta particles).
Interaction of photons with matter
As they pass through matter, photons interact with atoms. The type of interaction is a function of the energy of the photons and the atomic number ( Z ) of elements composing the matter.
Types of photon interactions in matter
In the practice of nuclear medicine, where gamma rays with energies between 50 keV and 550 keV are used, Compton scatteringis the dominant type of interaction in materials with lower atomic numbers, such as human tissue ( Z = 7.5). The photoelectric effectis the dominant type of interaction in materials with higher atomic numbers, such as lead ( Z = 82). A third type of interaction of photons with matter, pair production, only occurs with very high photon energies (greater than 1020 keV) and is therefore not important in clinical nuclear medicine. Figure 2.1depicts the predominant type of interaction for various combinations of incident photons and absorber atomic numbers.
Compton scattering
In Compton scattering the incident photon transfers part of its energy to an outer shell or (essentially) “free” electron, ejecting it from the atom. Upon ejection this electron is called the Compton electron. The photon, which has lost energy in the interaction, is scattered ( Figure 2.2) at an angle that depends on the amount of energy transferred from the photon to the electron. The scattering angle can range from nearly 0° to 180°. Figure 2.3illustrates scattering angles of 135° and 45°.
Photoelectric effect
An incident photon may also transfer its energy to an orbital (generally inner‐shell) electron. This process is called the photoelectric effectand the ejected electron is called a photoelectron( Figure 2.4). This electron leaves the atom with an energy equal to the energy of the incident gamma ray diminished by the binding energy of the electron. An outer‐shell electron then fills the inner‐shell vacancy and the excess energy is emitted as an X‐ray.
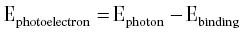
Table 2.1lists the predominant photon interactions in some common materials.
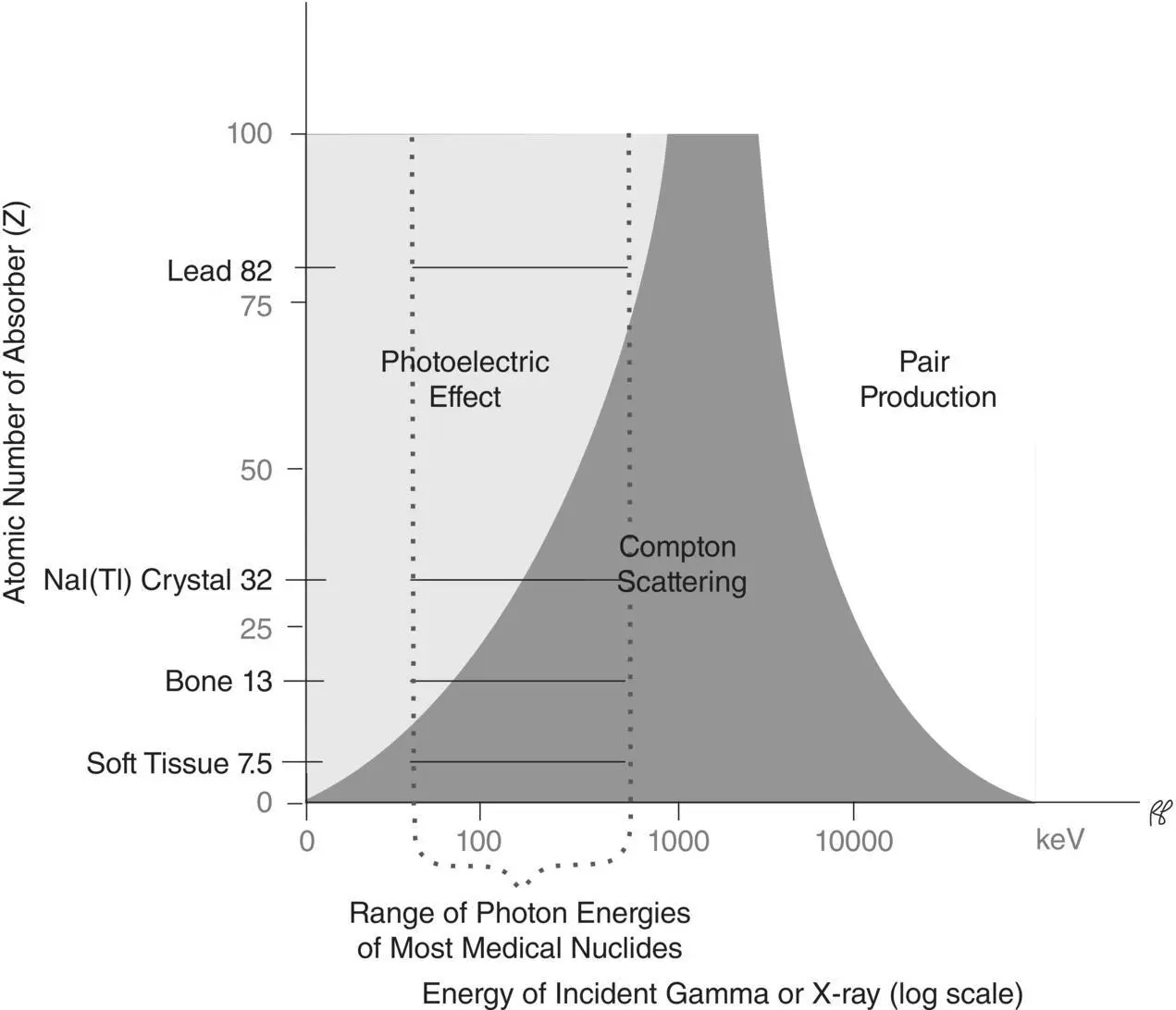
Figure 2.1 Predominant type of interaction for various combinations of incident photons and absorber atomic numbers.
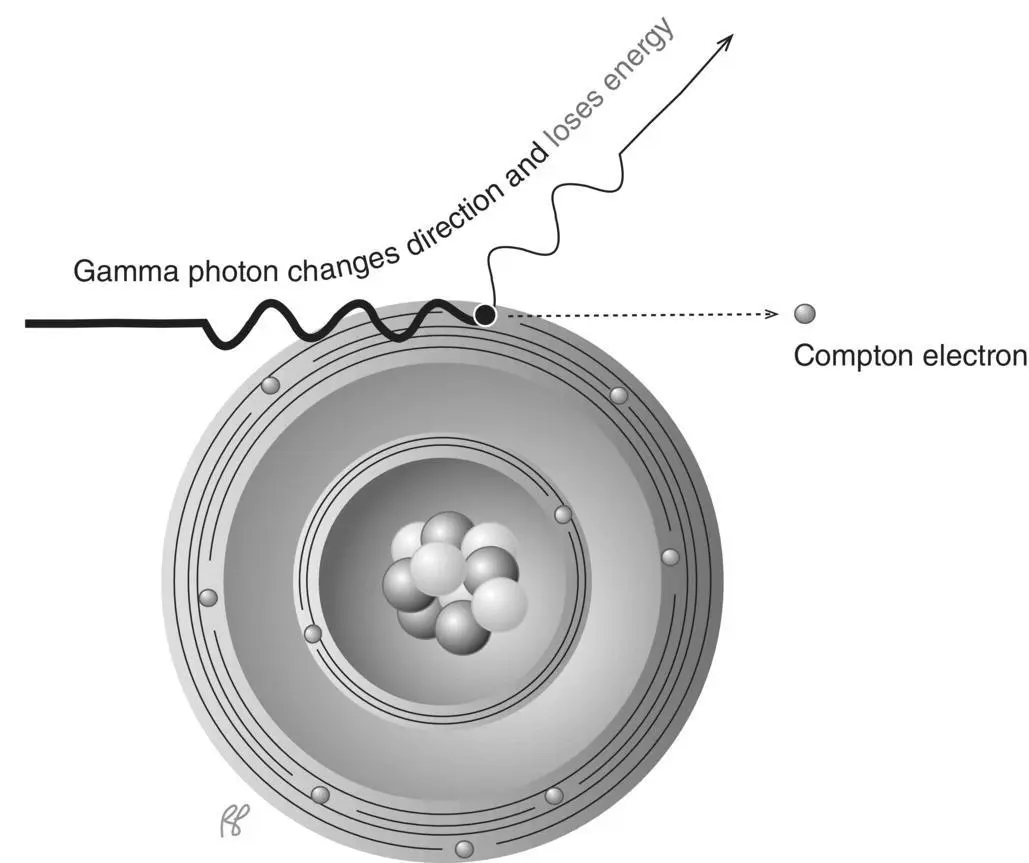
Figure 2.2 Compton scattering.
Attenuation of photons in matter
As the result of the interactions between photons and matter, the intensityof the beam(stream of photons), that is, the number of photons remaining in the beam, decreases as the beam passes through matter ( Figure 2.5). This loss of photons is called attenuation; the matter through which the beam passes is referred to as the attenuator. Specifically, attenuation is the ratio of intensity at the point the beam exits the attenuator, I out,to the intensity it had where it entered, I in.Attenuation is an exponential function of the thickness, x , of the attenuator in centimeters. That the function is exponential can be understood to mean that if half of the beam is lost in traversing the first centimeter of material, half of the remainder will be lost traversing the next centimeter, and so on. This resembles the exponential manner in which radioactivity decays with time. Expressed symbolically,
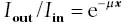
where μ , the linear attenuation coefficientis a property of the attenuator. When, as is usually the case, thickness is given in centimeters, the linear attenuation coefficient is expressed as “per centimeter” or “cm –1”. As might be expected, the linear attenuation coefficient is greater for dense tissue such as bone than for soft tissue such as fat. In general, the linear attenuation coefficient depends on both the energy of the photons and on the average atomic number ( Z ) and thickness of the attenuator. The lower the energy of the photons or the greater the average atomic number or thickness of the attenuator, the greater the attenuation ( Figure 2.6).
Читать дальшеИнтервал:
Закладка:
Похожие книги на «Essentials of Nuclear Medicine Physics, Instrumentation, and Radiation Biology»
Представляем Вашему вниманию похожие книги на «Essentials of Nuclear Medicine Physics, Instrumentation, and Radiation Biology» списком для выбора. Мы отобрали схожую по названию и смыслу литературу в надежде предоставить читателям больше вариантов отыскать новые, интересные, ещё непрочитанные произведения.
Обсуждение, отзывы о книге «Essentials of Nuclear Medicine Physics, Instrumentation, and Radiation Biology» и просто собственные мнения читателей. Оставьте ваши комментарии, напишите, что Вы думаете о произведении, его смысле или главных героях. Укажите что конкретно понравилось, а что нет, и почему Вы так считаете.












