Joseph J. Torres - Life in the Open Ocean
Здесь есть возможность читать онлайн «Joseph J. Torres - Life in the Open Ocean» — ознакомительный отрывок электронной книги совершенно бесплатно, а после прочтения отрывка купить полную версию. В некоторых случаях можно слушать аудио, скачать через торрент в формате fb2 и присутствует краткое содержание. Жанр: unrecognised, на английском языке. Описание произведения, (предисловие) а так же отзывы посетителей доступны на портале библиотеки ЛибКат.
- Название:Life in the Open Ocean
- Автор:
- Жанр:
- Год:неизвестен
- ISBN:нет данных
- Рейтинг книги:5 / 5. Голосов: 1
-
Избранное:Добавить в избранное
- Отзывы:
-
Ваша оценка:
- 100
- 1
- 2
- 3
- 4
- 5
Life in the Open Ocean: краткое содержание, описание и аннотация
Предлагаем к чтению аннотацию, описание, краткое содержание или предисловие (зависит от того, что написал сам автор книги «Life in the Open Ocean»). Если вы не нашли необходимую информацию о книге — напишите в комментариях, мы постараемся отыскать её.
Life in the Open Ocean: The Biology of Pelagic Species
Life in the Open Ocean: The Biology of Pelagic Species
Life in the Open Ocean — читать онлайн ознакомительный отрывок
Ниже представлен текст книги, разбитый по страницам. Система сохранения места последней прочитанной страницы, позволяет с удобством читать онлайн бесплатно книгу «Life in the Open Ocean», без необходимости каждый раз заново искать на чём Вы остановились. Поставьте закладку, и сможете в любой момент перейти на страницу, на которой закончили чтение.
Интервал:
Закладка:
We can arrive at a similar volume measurement for terrestrial systems by assuming that biologically useful space on land is defined by the height of a tall tree (0.05 km). Using 29% of the total surface area of the Earth for the terrestrial ecosystems and multiplying that by the height of the tree, we arrive at one useful‐volume estimate of 7.4 × 10 6km 3, which is only 0.5% of the volume in the oceans (cf. Herring 2002).
It is interesting to look at extremes. The highest point on Earth above sea level is Mt Everest at 8.55 km. The lowest point on Earth is in the Pacific Ocean, the Challenger Deep in the Marianas Trench near the Philippines at 10.93 km below sea level.
The total living space in the pelagic realm can also be compared with that in the terrestrial biome by evaluating surface area. The Hypsographic Curve of the world in Figure 1.1expresses graphically the amounts of the planet’s surface, by elevation, above and below sea level. From the Curve it is easy to see that over 60% of the Earth’s surface is covered by water of depths greater than 1000 m. Since 97% of all the water on Earth is contained in the oceans, the open sea at 1000 m deep and below is therefore the Earth’s “average environment”!
Freshwater in rivers and lakes accounts for about 1% of the Earth’s water; glaciers, polar ice, and groundwater contain about 2% more. The remainder of the Earth’s water is in the vastness of the oceans.
The Properties of Water
The physical properties of water do much to shape the Earth’s climate, while the density of water literally influences the shape of marine species. Water exhibits many unusual characteristics. It is the only compound that can be found in all three phases (solid, liquid, and gas) at the temperatures encountered at the Earth’s surface. Water has a high specific heat, which means that it must absorb a great deal of heat energy for its temperature to rise by 1 °C (1.0 cal g −1at 10 °C). Because it can absorb a lot of heat, it can also deliver a lot of heat to regions far removed from where the heat was absorbed, and ocean currents such as the Gulf Stream do just that. The climate of Great Britain, for example, would be far colder than it is were it not for the warm maritime influence of the Gulf Stream.
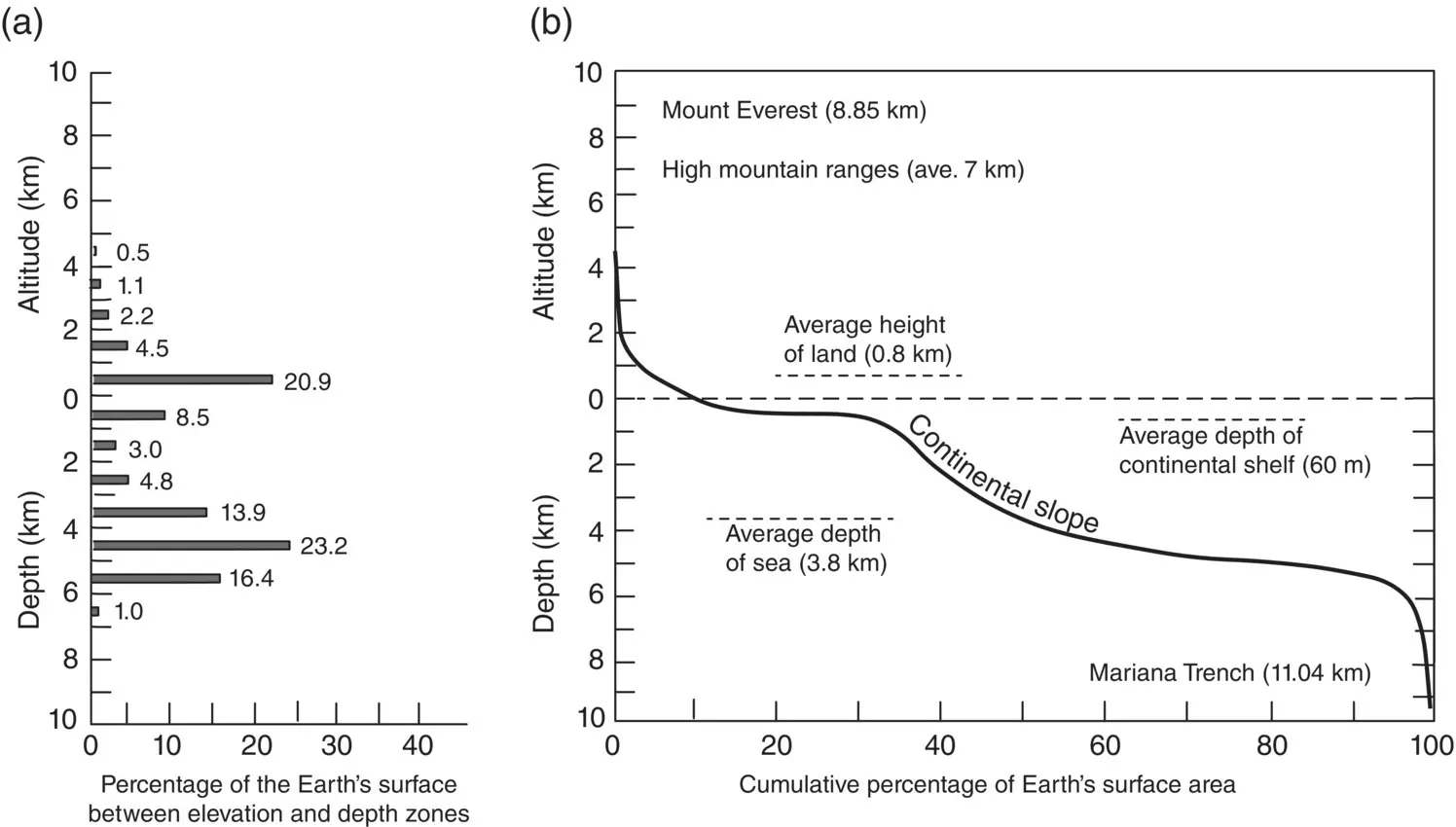
Figure 1.1 The Earth's surface area. (a) Earth’s surface area as a function of elevation and depth. (b) The Hypsographic Curve; cumulative percentage of earth's surface area as a function of elevation and depth.
Source: Gage and Tyler (1991), figure 2.4(p. 12). Reproduced with the permission of Cambridge University Press.
When water changes its physical state, a great deal of energy is gained or lost. When ice is formed, 80 cal g −1is liberated as the latent heat of fusion. This property is particularly important at the poles during the freezing and melting of sea ice. Similarly, 540 cal g −1must be applied to cause liquid water to evaporate (latent heat of evaporation) and an equal amount of energy is released upon condensation. Water’s freezing (0 °C) and boiling (100 °C) points are far higher than those of closely related compounds: H 2S, e.g. boils at about −59 °C.
Water has a high thermal conductivity (0.587 W m −1°K −1at 10 °C), so heat diffuses readily through it. When well mixed, like the wind‐mixed surface layer of the ocean, large volumes of water can be quite homogeneous in temperature. Water has a high surface tension, which makes it fairly “sticky.” Small volumes will form drops, capillary action will cause water to readily invade a small tube, and small water‐dwelling animals must contend with a fairly viscous environment.
Water’s most unusual characteristic by far is that it is less dense in its solid form than in its liquid phase. It is unique in the physical world in that regard, and it is because of that distinctive characteristic that ice floats. Pure water reaches its maximum density at 4 °C. Seawater does not reach maximum density until −3.5 °C (Vogel 1981), which is below its freezing point at −1.9 °C and ice crystals have already begun to form. When seawater freezes, the salt is excluded as a brine; the ice itself is fundamentally salt‐free.
Density
As Vogel (1981) observed, the concept of mass when applied to the inherent shapelessness of fluids is a bit awkward and for practical purposes is replaced by density. The density of pure water is about 830 times that of air, or about 1000 kg m −3at 0 °C and atmospheric pressure. It only varies by about 0.8% in density over the biological range of temperatures (0–40 °C) despite our attention to the fact that its maximum density is above its freezing point. Density of water is even less sensitive to pressure; it increases by only 0.5% with each kilometer of depth (Denny 1993).
Salinity alters the density of water considerably more than does temperature or pressure. The difference in density between freshwater and seawater is substantial. Salinity itself is the amount of dissolved material expressed in g per kg of seawater. The material consists mainly of salts; the principal salt is sodium chloride. The nicely intuitive definition of salinity as g per kg, or parts per thousand, has been replaced in some circles by the introduction of practical salinity. Practical salinity ( S p; UNESCO 1983) is the ratio of the electrical conductivity of a seawater sample and to a standard solution of potassium chloride. Since it is a ratio, practical salinity has no units. It is very close to actual salinity, though. To get back to actual salinity in g per kg from practical salinity, you use the following equation: S = 1.00510 S p(Bearman 1989).
The fact that water is far denser than air has its good points and bad points from the perspective of a swimming animal. On the plus side, it means that much less structural investment is required to support the weight of an organism in water than on land. A popular analogy compares a tree and a kelp of equal height above the substrate. Clearly, the kelp has far less energy invested in its 0.05 m diameter stipe than the tree has in its 0.5 m trunk. The principle works equally well for a jellyfish elegantly trailing its tentacles in the ocean or piled up in a soggy mass on the beach.
The aquatic medium provides buoyant support according to the difference in density between the body and the medium in which it is immersed. The weight of an object in water is described by the equation
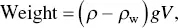
where ρ (rho) is the density of the object, ρ wis the density of water, g is the acceleration of gravity (9.8 m s −2), and V is the volume of the object in question. The expression ( ρ − ρ w) is the effective density or ρ eof our submerged body and determines whether it will float, remain suspended, or sink. Its effective weight in water is thus g · ρ e V , and it follows logically that a body will weigh more in air than in water, usually by 5‐ to 50‐fold (Denny 1993). Do not be guilty of synonymizing weight and mass. Mass is a scalar quantity measured in kilograms; weight is a force that is measured in newtons. You will notice that the mass of an object in water does not change, but its weight does.
Seawater at a salinity of 35‰ has a density of 1024 kg m −3, meaning that marine species enjoy more buoyant support from their medium than do their freshwater counterparts. Knowing what we know about relative weights in air and water, neutral buoyancy for marine species will be achieved with a density equal to that of seawater. Let us compare the density of some common biological materials. Mollusk shells at 2700 kg m −3are quite dense, providing protection and support for the soft tissues beneath but also assuring that they are most useful in bottom‐dwelling species. Cow bones are also quite dense, 2060 kg m −3, providing the skeletal support needed by a heavy animal in air. Neither structure is appropriate for a species concerned with remaining suspended in mid‐water, so the likelihood of cows invading the marine environment remains low. In contrast, muscle is 1050–1080 kg m −3, only about 5% higher than the density of seawater. Fats are slightly less dense than seawater, 915–945 kg m −3so they provide a source of static lift for marine species. It is instructive to note that small changes in an animal’s density can confer big advantages to its weight in water but would do little to affect its weight in air. The energetic advantages of neutral buoyancy have done much to influence how pelagic species are put together. In succeeding chapters, we shall explore buoyancies and mechanisms for achieving neutral buoyancy in open‐ocean taxa.
Читать дальшеИнтервал:
Закладка:
Похожие книги на «Life in the Open Ocean»
Представляем Вашему вниманию похожие книги на «Life in the Open Ocean» списком для выбора. Мы отобрали схожую по названию и смыслу литературу в надежде предоставить читателям больше вариантов отыскать новые, интересные, ещё непрочитанные произведения.
Обсуждение, отзывы о книге «Life in the Open Ocean» и просто собственные мнения читателей. Оставьте ваши комментарии, напишите, что Вы думаете о произведении, его смысле или главных героях. Укажите что конкретно понравилось, а что нет, и почему Вы так считаете.












