Cheanyeh Cheng - Enzyme-Based Organic Synthesis
Здесь есть возможность читать онлайн «Cheanyeh Cheng - Enzyme-Based Organic Synthesis» — ознакомительный отрывок электронной книги совершенно бесплатно, а после прочтения отрывка купить полную версию. В некоторых случаях можно слушать аудио, скачать через торрент в формате fb2 и присутствует краткое содержание. Жанр: unrecognised, на английском языке. Описание произведения, (предисловие) а так же отзывы посетителей доступны на портале библиотеки ЛибКат.
- Название:Enzyme-Based Organic Synthesis
- Автор:
- Жанр:
- Год:неизвестен
- ISBN:нет данных
- Рейтинг книги:3 / 5. Голосов: 1
-
Избранное:Добавить в избранное
- Отзывы:
-
Ваша оценка:
- 60
- 1
- 2
- 3
- 4
- 5
Enzyme-Based Organic Synthesis: краткое содержание, описание и аннотация
Предлагаем к чтению аннотацию, описание, краткое содержание или предисловие (зависит от того, что написал сам автор книги «Enzyme-Based Organic Synthesis»). Если вы не нашли необходимую информацию о книге — напишите в комментариях, мы постараемся отыскать её.
An insightful exploration of an increasingly popular technique in organic chemistry Enzyme-Based Organic Synthesis
Enzyme-Based Organic Synthesis
Enzyme-Based Organic Synthesis
Enzyme-Based Organic Synthesis — читать онлайн ознакомительный отрывок
Ниже представлен текст книги, разбитый по страницам. Система сохранения места последней прочитанной страницы, позволяет с удобством читать онлайн бесплатно книгу «Enzyme-Based Organic Synthesis», без необходимости каждый раз заново искать на чём Вы остановились. Поставьте закладку, и сможете в любой момент перейти на страницу, на которой закончили чтение.
Интервал:
Закладка:
Source: Based on Nelson and Cox [9].
| Coenzyme | Chemical groups transferred | Dietary precursor in mammals |
| Biocytin | CO 2 | Biotin |
| Coenzyme A | Acyl group | Pantothenic acid and other compounds |
| 5’‐Deoxyadenosylcobalamin (coenzyme B 12) | H atoms and alkyl groups | Vitamin B 12 |
| Flavin adenine dinucleotide | Electrons | Riboflavin (vitamin B 2) |
| Lipoate | Electrons and Acyl groups | Not required in diet |
| Nicotinamide adenine Dinucleotide | Hydride ion (:H −) | Nicotinic acid (niacin) |
| Pyridoxal phosphate | Amino groups | Pyridoxine (vitamin B 6) |
| Tetrahydrofolate | One‐carbon groups | Folate |
| Thiamine pyrophosphate | Aldehydes | Thiamine (vitamin B 1) |
For some enzymes, a coenzyme is required for their activity. A coenzyme or metal ion that is bound to the enzyme protein at the active site is called a prosthetic group. The protein part of such an enzyme is called the apoenzyme or apoprotein, and the entire enzyme is called a holoenzyme. Most of these cofactors are relatively unstable molecules. We will consider various coenzymes throughout the text in more detail for those related enzyme‐catalyzed reactions.
1.4 Molecular Recognition and Enzyme Specificity
The science by which molecules interact via geometric orientations of atoms is called molecular recognition. The specific geometry of a molecule controls how it will react with other substances. Nearly, all enzymes are made up of more than 100 amino acid residues. However, an enzyme binds a substrate molecule at the catalytic active site that is just a small pocket or cleft region of the enzyme. The 3D structure of the active site is surrounded by amino acid side chains that come from different parts of the linear amino acid sequence. Water is usually excluded unless it is a reactant. The nonpolar character of much of the cleft enhances the binding of substrate. However, the cleft may also contain some polar residues that create a microenvironment essential for catalysis and extraordinary enzyme specificity. The specificity of binding of atoms in a substrate to the active site leads the proposed lock‐and‐key model of interaction between the substrate and enzyme by Emil Fischer in 1894 that points out the binding of a substrate to an enzyme just like the relationship of a key to a lock. The lock‐and‐key model is now modified to the induced fit model by Daniel Koshland in 1958 with the evident that the enzyme and substrate must adjust to fit one another to take up a configuration to stabilize the transition state [10, 11].
However, to assure the necessary geometric accuracy of the substrate binding and the orientation of catalytic functional group for enzyme interaction, the number of specific binding sites or points needed between the substrate and the active site of enzyme depends on the size of a molecule. For a large molecule such as glycyl tyrosine, a dipeptide, can bind carboxypeptidase A through total of five points at the active site [10, 12]: the electrostatic force, two hydrogen bonds, the hydrophobic interaction, and the coordination bonding to perform the hydrolysis of peptide bond of glycyl tyrosine. For small molecule such as carbon dioxide and water can coordinative bind carbonic anhydrase with only one point through the cofactor zinc ion and react to form bicarbonate or the reversible reaction [10]. Another example for small molecule is the binding of a covalent adduct formed between pyruvate and nicotinamide adenine dinucleotide (NAD +) to lactate dehydrogenase (LDH) to produce lactate in which only two binding points, namely, the carbonyl group and the carboxylate group, of pyruvate are used to bind with the LDH [12]. The number of binding sites of an enzyme to the substrate is important in determining the type of enzyme specificity. The molecular recognition for enzyme specificity has been categorized into three major types of specificities: substrate specificity, regiospecificity, and stereospecificity [13].
1.4.1 Substrate Specificity
As a catalyst, the most distinguished property of enzyme is its substrate specificity. This kind of specificity determines its unique biological function. Many enzymes catalyze only one particular biological substrate. Enzymes with this kind of specificity are, therefore, called having absolute substrate specificity. For example, glucose‐6‐phosphatase binds only glucose‐6‐phosphate (G6P) to hydrolyze catalytically the phosphate moiety from the G6P. Therefore, the substrate specificity of glucose‐6‐phosphatase is absolute substrate specificity. Other enzymes possess a broader substrate specificity called relative group specificity that takes a group of biological molecules of similar chemical structure as substrate. As an example, acid and alkaline phosphatase can form phosphate ester bond for a variety of substrates. Therefore, acid and alkaline phosphatase presents wider relative group specificity.
Whether an enzyme has absolute substrate specificity or relative group specificity depends on the number of binding sites of the enzyme–substrate complex. Figure 1.1illustrates the number of binding sites of an enzyme to the substrate makes the enzyme absolute substrate specificity or relative group substrate specificity. There is only one specific molecule that can attach the enzyme with four binding sites as shown in Figure 1.1a that gives absolute substrate specificity for the enzyme. The number of biding sites for the enzyme with substrate shown in Figure 1.1a–c is three, two, and one, respectively, that makes a variety of different groups available for the unbound group in the substrate to produce a relative group specificity for the enzyme. The substrate specificity of enzyme brings the advantage of reducing the possible side reactions, thus eliminating the laborious purification and separation works.
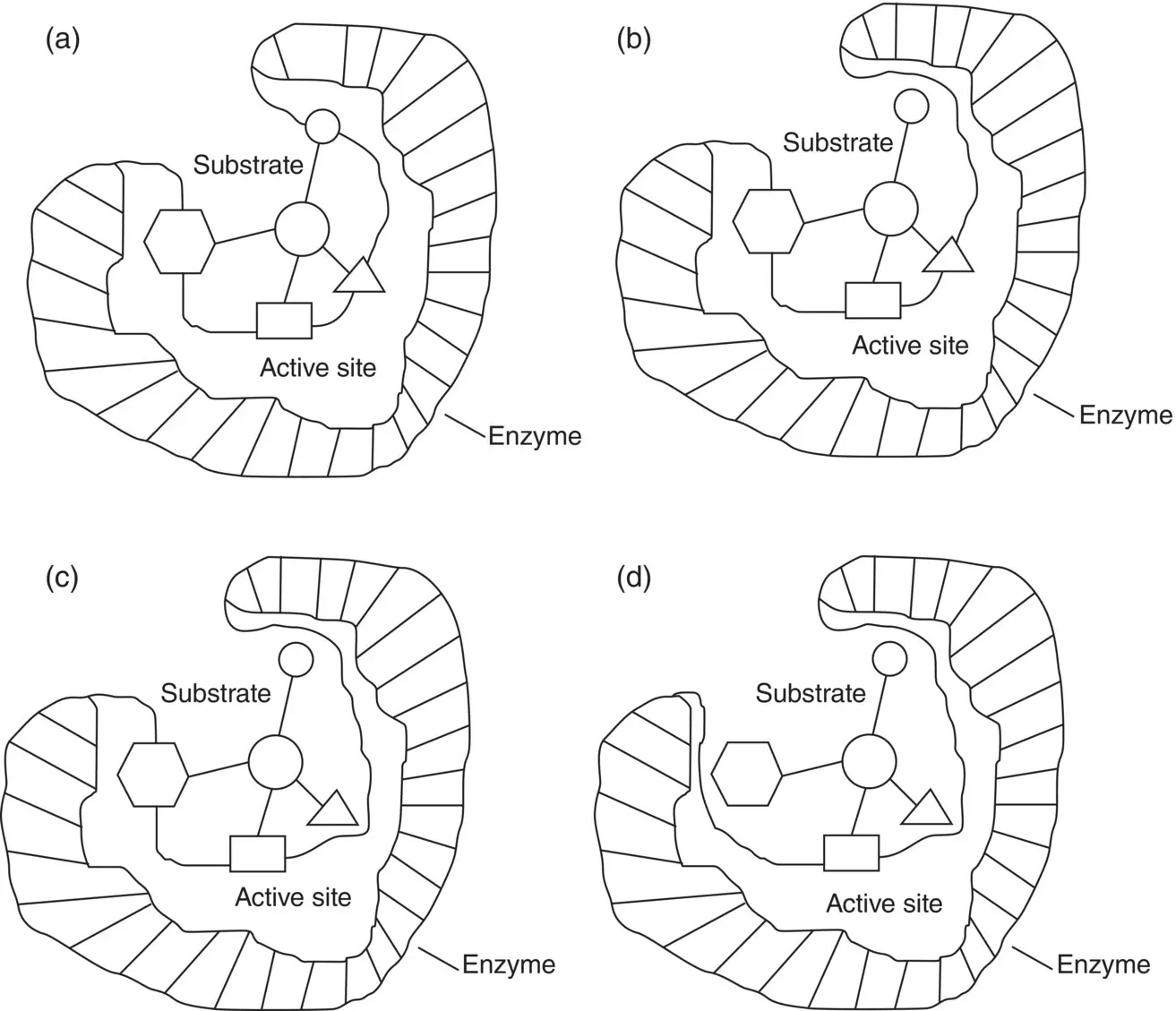
Figure 1.1 The number of binding sites of a substrate with enzyme determines the type of enzyme specificity: (a) Absolute substrate specificity of enzyme, (b–d) relative group specificity of enzyme.
1.4.2 Regiospecificity
Some of the enzymes may selectively catalyze one functional group at certain region of the molecule among several similar functional groups located at different regions of the same molecule. This kind of substrate specificity is called regiospecificity or diastereospecificity [13]. Example of regiospecificity in organic synthesis has been found since 1986 that the regioselective deacylation of methyl 2,3,4,6‐tetra‐ O ‐acyl‐D‐hexopyranosides gives the 6‐OH derivatives in high yields using the lipase from Candida cylindracea [14]. The enzyme‐catalyzed regioselective O ‐acylation of ribo‐, arabino‐, xylo‐, rhamnopyranosides, and aryl pyranosides is reviewed, and the methodology is applied to the total synthesis of the naturally occurring rhamnopyranoside by Bashir et al [15]. Regioselective biotransformation of dinitrile compounds 2‐, 3‐, and 4‐(cyanomethyl) benzonitrile can be performed by whole bacterium cell Rhodococcus rhodochrous to the corresponding 2‐(cyanophenyl) acetic acid, 3‐ or 4‐(cyanomethyl) benzoic acid with high yield [16]. Whole cell bacterium Bacillus cereus has also been used for regioselectively converting 2‐phenylenediamine to 2‐aminoacetanilide with a76% molar yield [17]. Other than bacterium, monooxygenase in the phytopathogenic fungi Colletotrichum gloeosporioides and Botrytis cinerea has been found having the ability of regioselective hydroxylation of the C‐H bonds to yield the corresponding diols [18]. Enzyme in cultured plant cells of Phytolacca Americana can reduce, and regioselectively hydroxylate and glucosylate, raspberry ketone and zingerone to their β‐glycosides [19]. Ginkgo biloba cell suspension cultures were used to regio‐ and stereoselectively convert sinenxan A, 2α,5α,10β,14β‐tetra‐acetoxy‐4(20), 11‐taxadiene, a taxoid isolated from callus tissue cultures of Taxus spp., in Taxol ®synthesis [20]. The regioselective oxidation of (–)‐verbenone, an important component of the essential oil from rosemary, to (–)‐10‐hydroxyberbenone with human liver microsomes has been investigated by Miyazawa et al [21]. Although regioselective oxidation of terpenoids is difficult by chemical methods, regioselective oxidation of (+)‐ and (–)‐citronellene was recently performed with Spodoptera litura , a larvae of common cutworm, to (2 R ,3 S )‐3,7‐dimethyl‐6‐octene‐1,2‐diol (yield: 89.7%) and (2 S ,3 R )‐3,7‐dimethyl‐6‐octene‐1,2‐diol (yield: 56.3%) [22]. These examples of enzyme‐catalyzed regiospecificity clearly elucidates that the specific enzyme–substrate binding configuration at the active site allows only one of several similar function groups in different regions of the substrate molecule reacts to produce the product. The number of binding points of enzyme–substrate complex for regioselective molecular recognition must be a “multi‐point” binding case to match the complexity of the substrate molecule.
Читать дальшеИнтервал:
Закладка:
Похожие книги на «Enzyme-Based Organic Synthesis»
Представляем Вашему вниманию похожие книги на «Enzyme-Based Organic Synthesis» списком для выбора. Мы отобрали схожую по названию и смыслу литературу в надежде предоставить читателям больше вариантов отыскать новые, интересные, ещё непрочитанные произведения.
Обсуждение, отзывы о книге «Enzyme-Based Organic Synthesis» и просто собственные мнения читателей. Оставьте ваши комментарии, напишите, что Вы думаете о произведении, его смысле или главных героях. Укажите что конкретно понравилось, а что нет, и почему Вы так считаете.












