1 ...6 7 8 10 11 12 ...30 The most magnificent specificity of enzyme is its distinguishable ability for only one enantiomeric structure of racemic substrate molecules. The molecular recognition of an enzyme for enantiomeric molecules is called enantiospecificity or stereospecificity. The stereospecificity is an intrinsic property of enzyme which is due to the chirality of active site of the enzyme. Except for a few cases, all enzymes are chiral catalysts because they all made from L‐amino acids, thus the binding of asymmetric substrate at the active site is stereoselective. Since the stereospecificity of enzyme involves enzyme–substrate complex formation with only one enantiomer of a racemate, only one product is formed from the enzyme‐catalyzed reaction. Therefore, enzyme‐catalyzed reactions are in great favor of the organic asymmetric synthesis [23].
The theoretical explanation for the stereospecificity of enzyme was based on the rationale of three‐point attachment rule [24, 25]. This rule suggests that at least three different binding points should occur between enzyme and substrate at the active site to make recognition for the correct stereostructure of substrate molecule as illustrated in Figure 1.2. However, the three‐point attachment is not strict for the stereospecificity of enzyme to asymmetric molecules. As shown in Figure 1.3, the recognition of the stereostructure of asymmetric molecules with enzyme can be accessed by two‐point binding of enzyme–substrate complex, under the situations that the stability of the enzyme–substrate complex is greatly influenced by the formation of two different 3D tetrahedral structure of enzyme–substrate complex or the ability of interaction for the two unbinding groups of the substrate with enzyme at the active site is obviously different. With only two‐point binding of the enzyme–substrate complex, one of the two substrate enantiomers possessing greater stability of the enzyme–substrate complex will have enough time to react to form product, but this situation will not happen for the other substrate enantiomer.
The preparation of enantiopure compounds is highly demanded by industries, particularly, in pharmaceutical and agrochemical applications because of the different biological activities of drug enantiomers. As a result of advances in asymmetric synthesis and separation technologies, there were many cases in using the stereospecificity of enzyme for the production of single enantiomer to allow the study of its pharmacodynamics and pharmacokinetic properties. For example, in the case of total synthesis of D‐biotin, the novel enantioselective synthesis of the optically active (3 aS ,6 aR )‐lactone (the key D‐biotin intermediate) was through kinetic resolution by inexpensive microbial lipase instead of pig liver esterase [26]. In Scheme 1.4, optically active (3 aS ,6 aR )‐lactone 1was enantioselectively produced with high enantiomeric excess.

Figure 1.2 Three‐point attachment rule shows that only one enantiomer of the asymmetric molecule can successfully bind with enzyme at the active site to produce the stereospecificity of enzyme.
(ee > 98%) and conversion ratio (≥ 40%) by dry microbial cells of Aspergillus oryzae WZ007 on racemic acid 1(1,3‐dibenzyl‐5‐(hydeoxymethyl)‐2‐oxo‐4‐imidazolidinecarboxylic acid) that was obtained via chemical hydrolysis of racemic lactone 1.
Recently, an indirect strategy was used for the synthesis of ( R )‐phenylephrine (an α 1‐adrenergic receptor agonist) that is widely used in over‐the‐counter drugs to treat the common cold. An amino alcohol dehydrogenase gene isolated from Rhodococcus erythropolis BCRC 10909 was expressed in Escherichia coli NovaBlue, which is able to convert 1‐(3‐hydroxyphenyl)‐2‐(methylamino) ethanone (HPMAE) to ( S )‐phenylephrine with more than 99% enantiomeric excess (ee) and 78% yield as shown in Scheme 1.5[27]. The ( S )‐phenylephrine was subsequently converted to ( R )‐phenylephrine by Walden inversion reaction [28]. Since enantiopure vicinal diols are useful and valuable intermediate for pharmaceutical production, a simple and green method for preparing several enantiopure 1,2‐diols was developed via regio‐ and stereoselective concurrent oxidations of the racemates with microbial cell Sphingomonas sp. HXN‐200.

Figure 1.3 The stereospecificity of enzyme for two substrate enantiomers by two‐point binding.
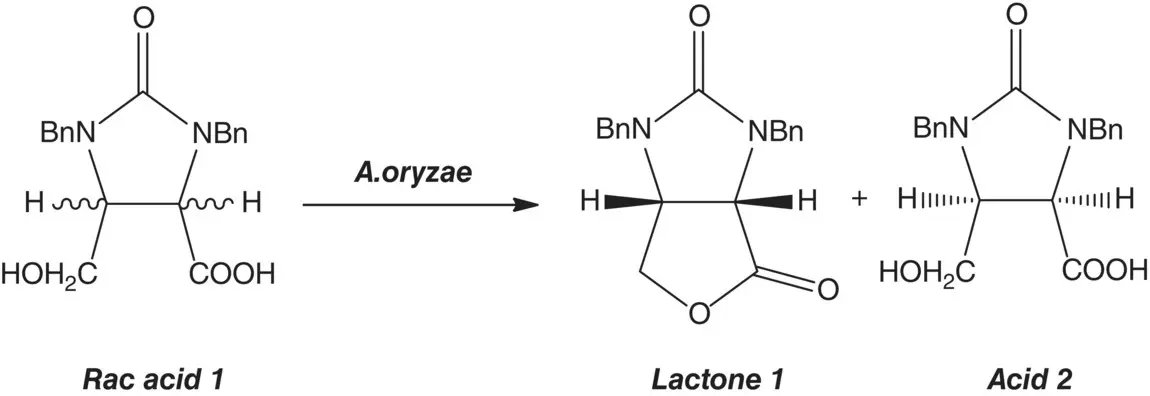
Scheme 1.4 Enantioselective synthesis of (3 aS ,6 aR )‐lactone.
As shown in Scheme 1.6, concurrent biooxidations of racemic 3‐ O ‐benylglycerol 1with resting cells gave ( S )‐ 1in 99.2% enantiomeric excess (ee) and 32% yield. Similar biooxidations of racemic 1‐(4‐chlorophenyl)‐1,2‐ethanediol 2, 1‐(4‐methylp henyl)‐1,2‐ethanediol 3, and phenyl‐1,2‐ethanediol 4gave ( R )‐ 2in 98.4% ee and 48% yield, ( R )‐ 3in 99.6% ee and 45% yield, and ( R )‐ 4in 98.7% ee and 36% yield, respectively [29].
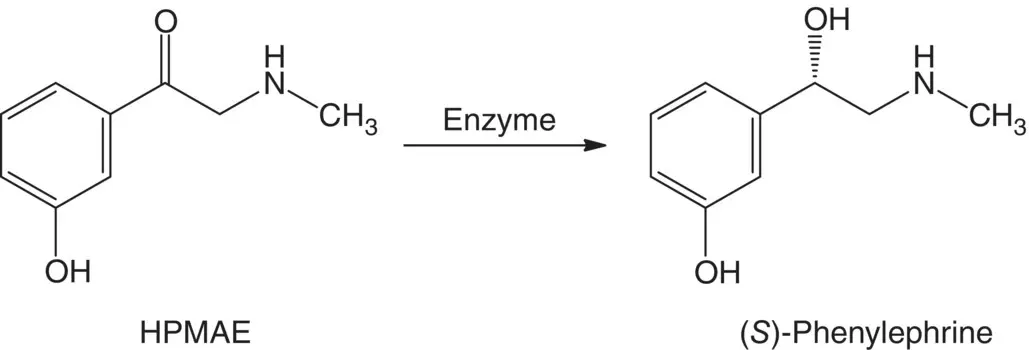
Scheme 1.5 The enantioselective conversion of HPMAE to ( S )‐phenylephrine.
Source: Lin et al. [27].
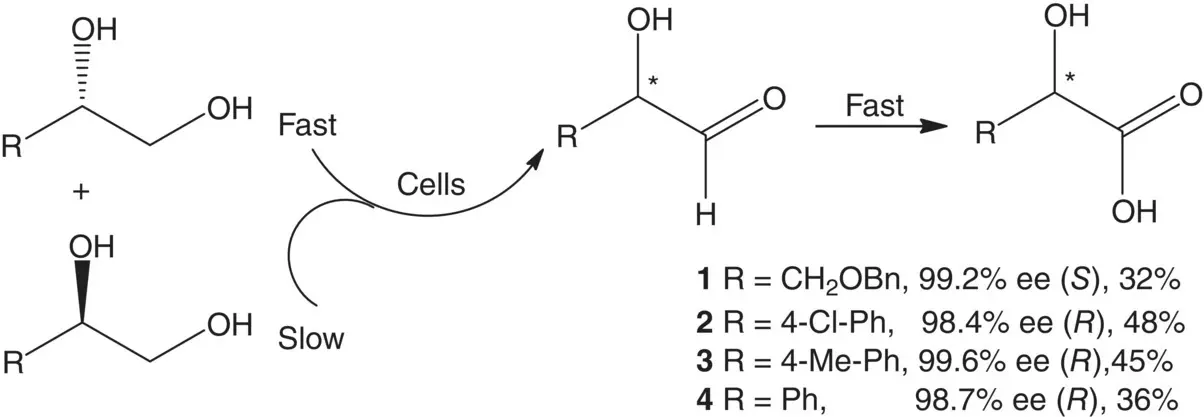
Scheme 1.6 Regio‐ and stereoselective concurrent oxidations of racemic vicinal diols to enantiopure 1,2‐diols.
1.5 Enzyme Classes and Nomenclature
Since Duclaux proposed that all enzymes should give the suffix “ase” for an easy recognition [3], the suffix “ase” has been added to the name of many enzymes according to their substrate or to a word or phrase for describing the activity. For example, glucose oxidase catalyzes the oxidation of glucose to produce gluconolactone, and cellulase catalyzes the hydrolysis of cellulose to form glucose. However, enzymes such as pepsin and trypsin have names that do not relate with their substrates or functions. Because more and more enzymes are discovered accompanied with the progress of scientific researches, the name of new enzyme may have two or more names, or two different enzymes may be given the same name. To avoid the ambiguity for naming enzymes, a systematic method for naming and classifying enzymes should be used and agreed globally.
In 1960s, the Commission on Enzyme Nomenclature was formed by International Union of Biochemistry (IUB) to classify enzymes into six major classes according to the type of reaction catalyzed as indicated in Table 1.4[2, 9, 30]. Each of the six major classes is further divided into subclasses and subgroups.
Читать дальше

















