Spiro Compounds
Здесь есть возможность читать онлайн «Spiro Compounds» — ознакомительный отрывок электронной книги совершенно бесплатно, а после прочтения отрывка купить полную версию. В некоторых случаях можно слушать аудио, скачать через торрент в формате fb2 и присутствует краткое содержание. Жанр: unrecognised, на английском языке. Описание произведения, (предисловие) а так же отзывы посетителей доступны на портале библиотеки ЛибКат.
- Название:Spiro Compounds
- Автор:
- Жанр:
- Год:неизвестен
- ISBN:нет данных
- Рейтинг книги:3 / 5. Голосов: 1
-
Избранное:Добавить в избранное
- Отзывы:
-
Ваша оценка:
- 60
- 1
- 2
- 3
- 4
- 5
Spiro Compounds: краткое содержание, описание и аннотация
Предлагаем к чтению аннотацию, описание, краткое содержание или предисловие (зависит от того, что написал сам автор книги «Spiro Compounds»). Если вы не нашли необходимую информацию о книге — напишите в комментариях, мы постараемся отыскать её.
A comprehensive treatment of the latest research in, and applications of, spiro compounds Spiro Compounds: Synthesis and Applications
Spiro Compounds
Spiro Compounds — читать онлайн ознакомительный отрывок
Ниже представлен текст книги, разбитый по страницам. Система сохранения места последней прочитанной страницы, позволяет с удобством читать онлайн бесплатно книгу «Spiro Compounds», без необходимости каждый раз заново искать на чём Вы остановились. Поставьте закладку, и сможете в любой момент перейти на страницу, на которой закончили чтение.
Интервал:
Закладка:
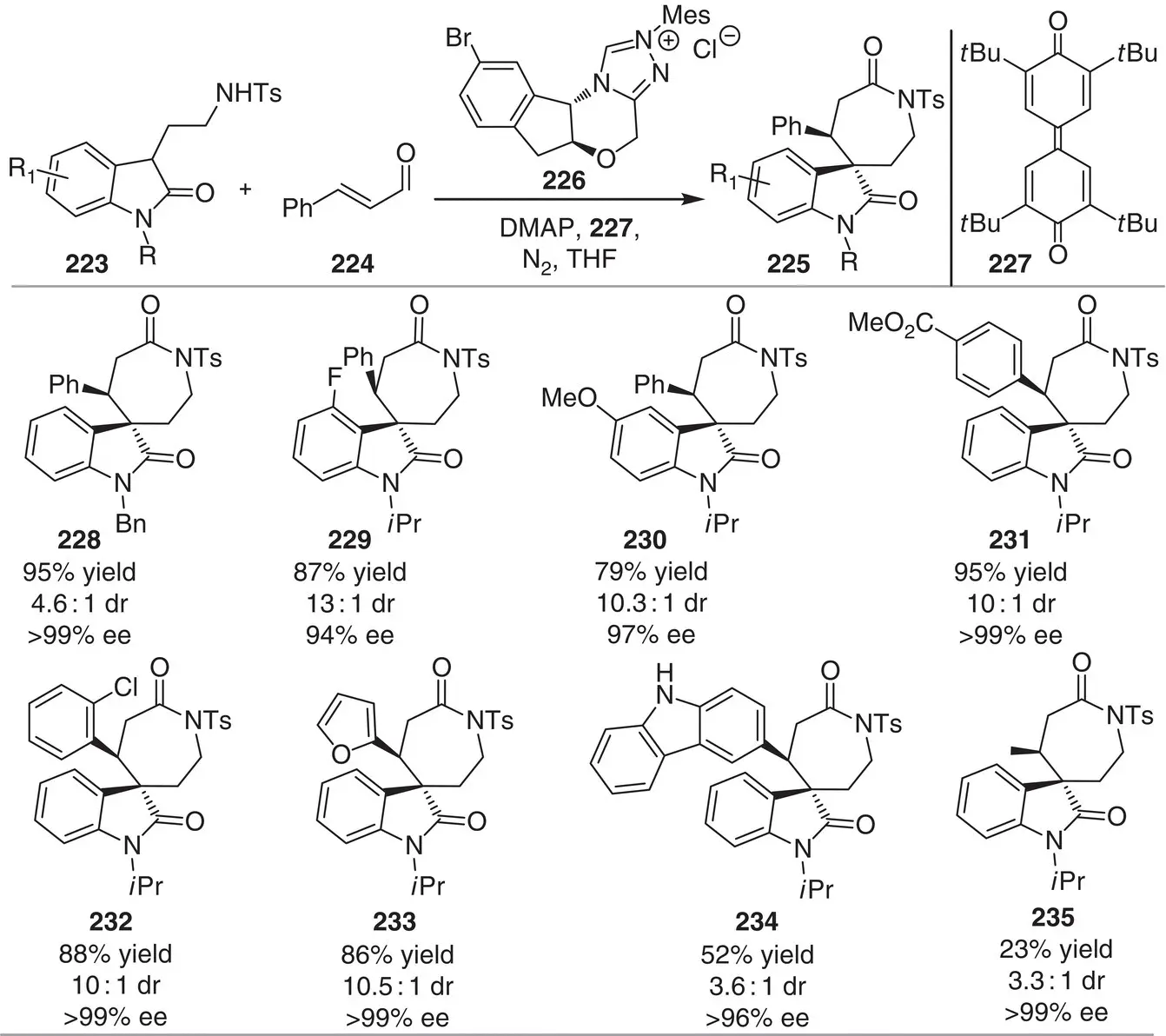
Scheme 3.21 NHC‐catalyzed [4+3] annulation of oxotryptamines with enals to access enantioenriched spiro‐ϵ‐lactam oxindoles.
Source: Modified from Liu et al. [33].
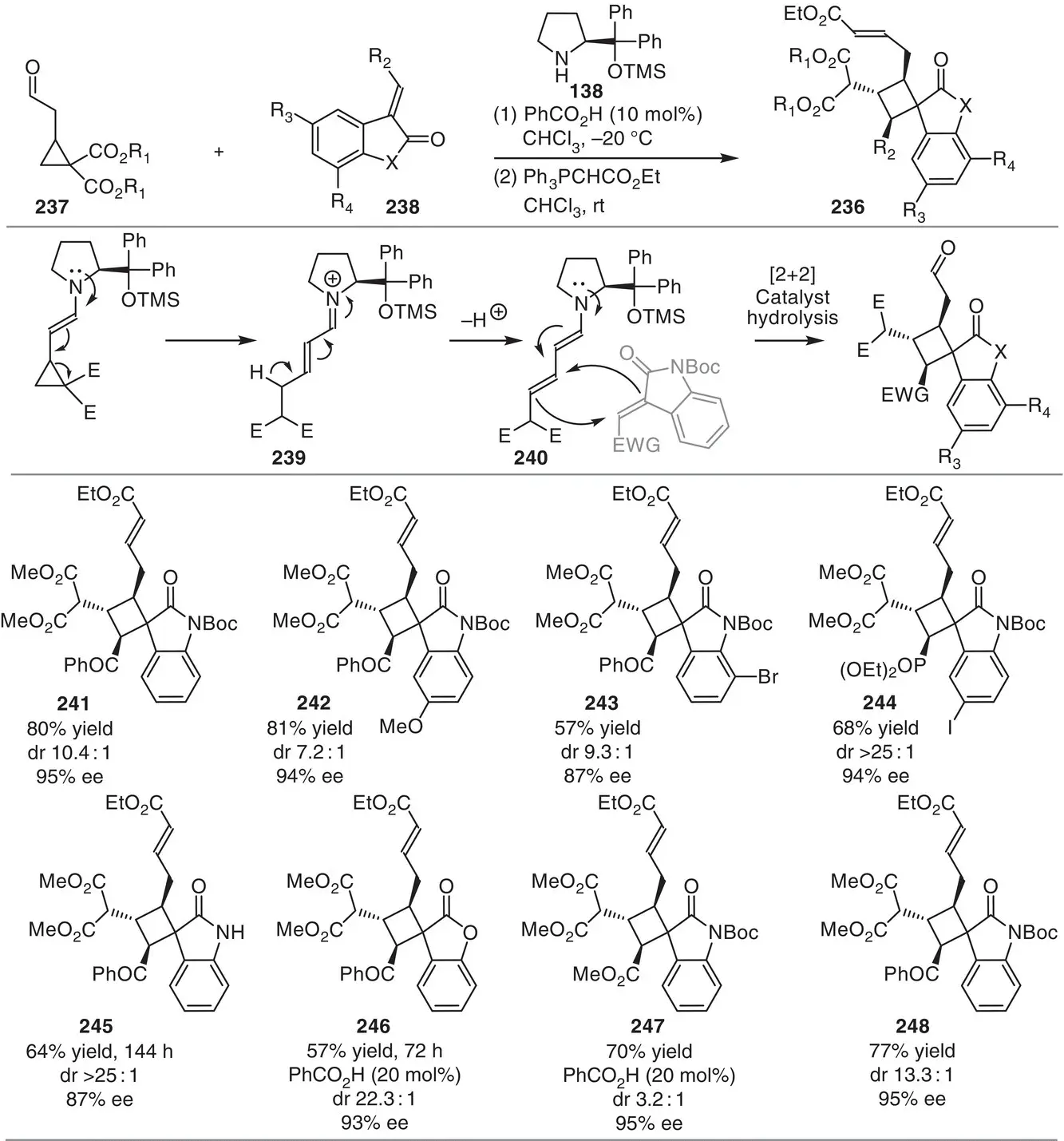
Scheme 3.22 Organocatalytic enamine‐activation of cyclopropanes for highly stereoselective formation of spiranic cyclobutanes.
Source: Modified from Halskov et al. [34].
The development of switchable intermolecular reactions that afford divergent reaction pathways is an attractive and powerful strategy to build up structural and stereogenic diversity in drug discovery. In this context, Ouyang, Chen, and coworkers reported an aminocatalytic divergent cycloaddition toward the formation of two different types of spirocompounds 249and 250using the same set of substrates ( Scheme 3.23) [35]. By simply changing the cocatalyst, the reaction pathway is switched from trienamine to dienamine activation mode. On the one hand, the in situ ‐generated 4‐aminofulvenes act as 6π partners in the γ,β´‐regioselective [6+2] cycloaddition with activated 2π alkenes under the catalysis of 252or 253and salicylic acid 254. On the other hand, the α,γ‐regioselective [4+2] cycloaddition reaction is promoted with the catalytic system 255/2‐mercaptobenzoic acid 256. In the latter case, the β′‐regioselective sulfur addition of thiol 256to 251gives access to the enone 257that after condensation with the chiral amine forms the 4π dienamine component in the reaction with activated alkenes. The [6+2] cycloaddition reaction mode provides fused bicycles linked to a pyrrolidinone ring 250with five contiguous stereogenic centers (28 examples) while the [4+2] pathway affords spiro‐bridged cyclic scaffold 249(22 examples).
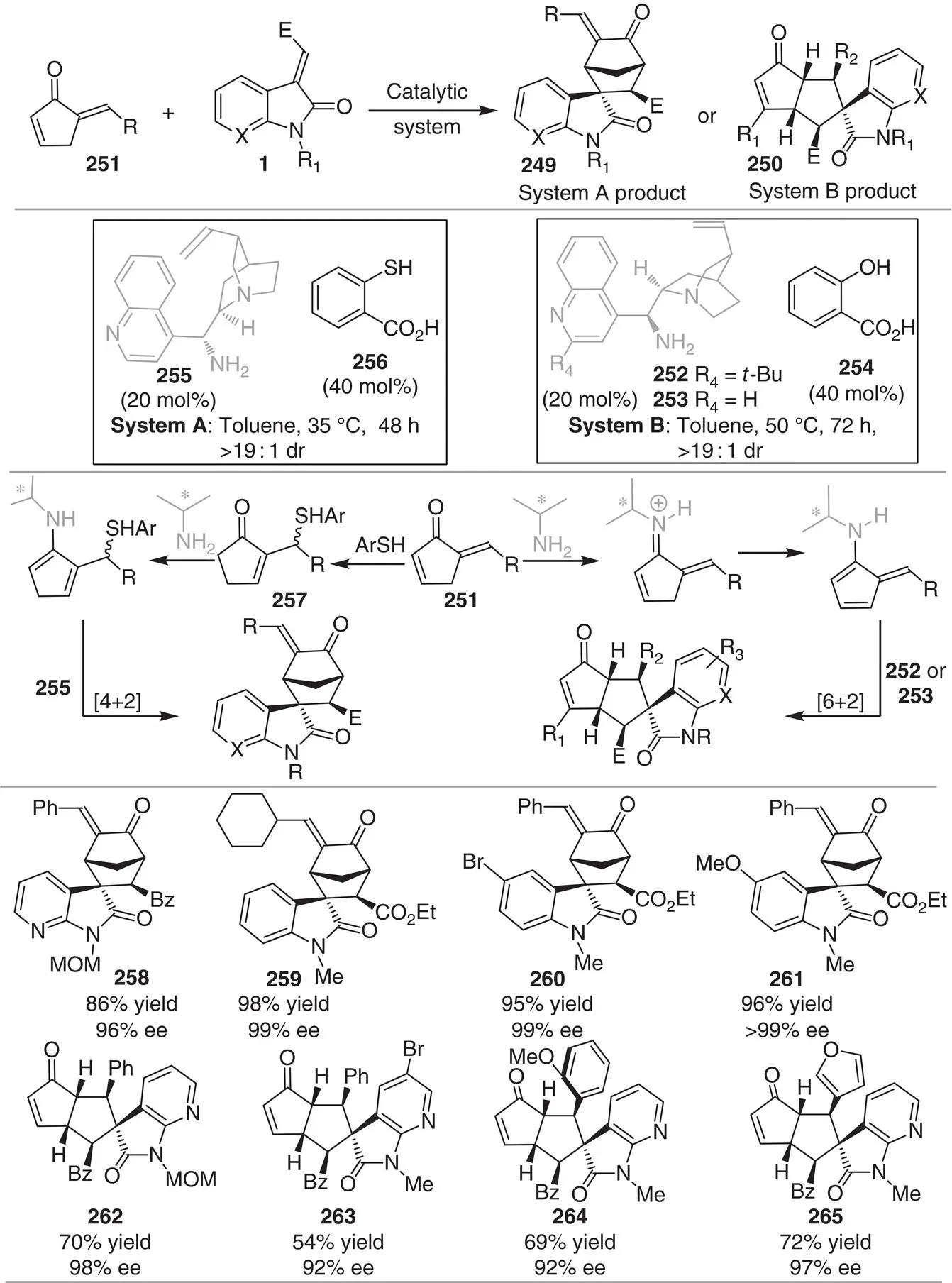
Scheme 3.23 Chiral primary amine‐catalyzed regiodivergent asymmetric cycloadditions.
Source: Modified from Zhou et al. [35].
3.3.4 Organocatalytic Miscellaneous Strategies to Construct Spiro Compounds
The group of Tu reported the enantioselective synthesis of spiro[4.4]nonane‐1,6‐diones 266, which represents the first direct example for the asymmetric synthesis of cyclopentanones using a Nazarov cyclization/semipinacol‐rearrangement sequence ( Scheme 3.24) [36]. Substrate design revealed key for the successful development of the present strategy, where a cyclobutanol motif is connected to the α‐position of the cyclization precursor to induce the 1,2‐rearrangement. This tandem process is promoted by means of a chiral N ‐triflylphosphoramide catalyst 268to construct complex spirocompounds with four consecutive stereocenters in excellent stereocontrol (15 examples, 93 : 7–>99 : 1 dr, 84–97% ee). Computational calculations were performed to understand the reaction mechanism as well as the stereochemical outcome. The reaction begins with the formation of a hydrogen‐bond complex between the substrate and the catalyst. Then, the substrate is protonated by the catalyst and can be regarded as a pentadienyl cation, which then undergoes 4π‐conrotatory electrocyclization via TS1. The Nazarov reaction step is irreversible, since the transition state TS2 following the ring expansion event is much lower in energy. Then, the intermediate 269undergoes a [1, 2] migration via TS2 to generate the intermediate 270that is finally protonated to afford the spiranic product 266.
In other example of Bronsted acid catalysis, Tan and coworkers developed an enantioselective strategy for the construction of axially chiral compounds 277( Scheme 3.25) [37]. This important class of spirocompounds is widely found in materials, organocatalysts, and ligands. The enantioselective intramolecular cyclization of ketals 278to afford the SPINOL derivatives 277is successfully catalyzed by the chiral phosphoric acid 279. Various ketals with different substitution patterns, including electron‐donating and electron‐withdrawing groups at the aryl ring, performed smoothly, affording the corresponding products 281–284in high enantioselectivities and good to excellent yields (90−96% ee, 62−95% yield, conditions a). In the case of ketals bearing aromatic groups at the ortho position, the catalyst 280(conditions b) was necessary to obtain the corresponding products. Remarkably, the catalytic system can be used at gram‐scale and the catalyst loading can be decreased as little as 0.1 mol%, despite higher temperature and prolonged reaction time are necessary.
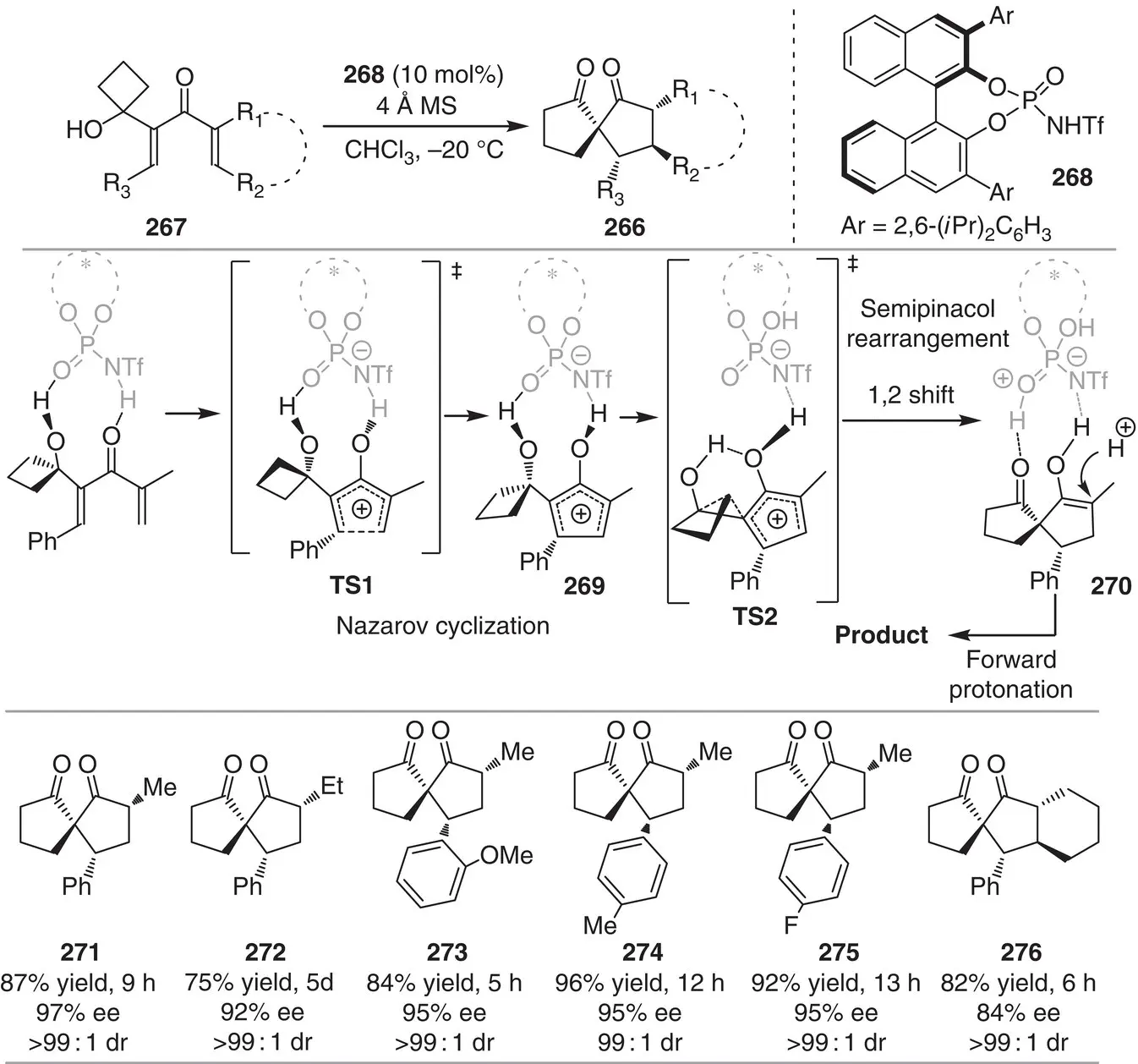
Scheme 3.24 Organocatalytic asymmetric tandem Nazarov cyclization/semipinacol rearrangement.
Source: Modified from Yang et al. [36].
On the basis of mechanistic experiments, the authors propose that the reaction may initially form the intermediate 289, in situ generated from 288, which further proceed through the active o ‐QM intermediate 290to deliver the SPINOL derivative. The excellent stereocontrol was attributed to the simultaneous interaction between the bifunctional phosphoric acid and intermediate 291via hydrogen‐bonding interactions.
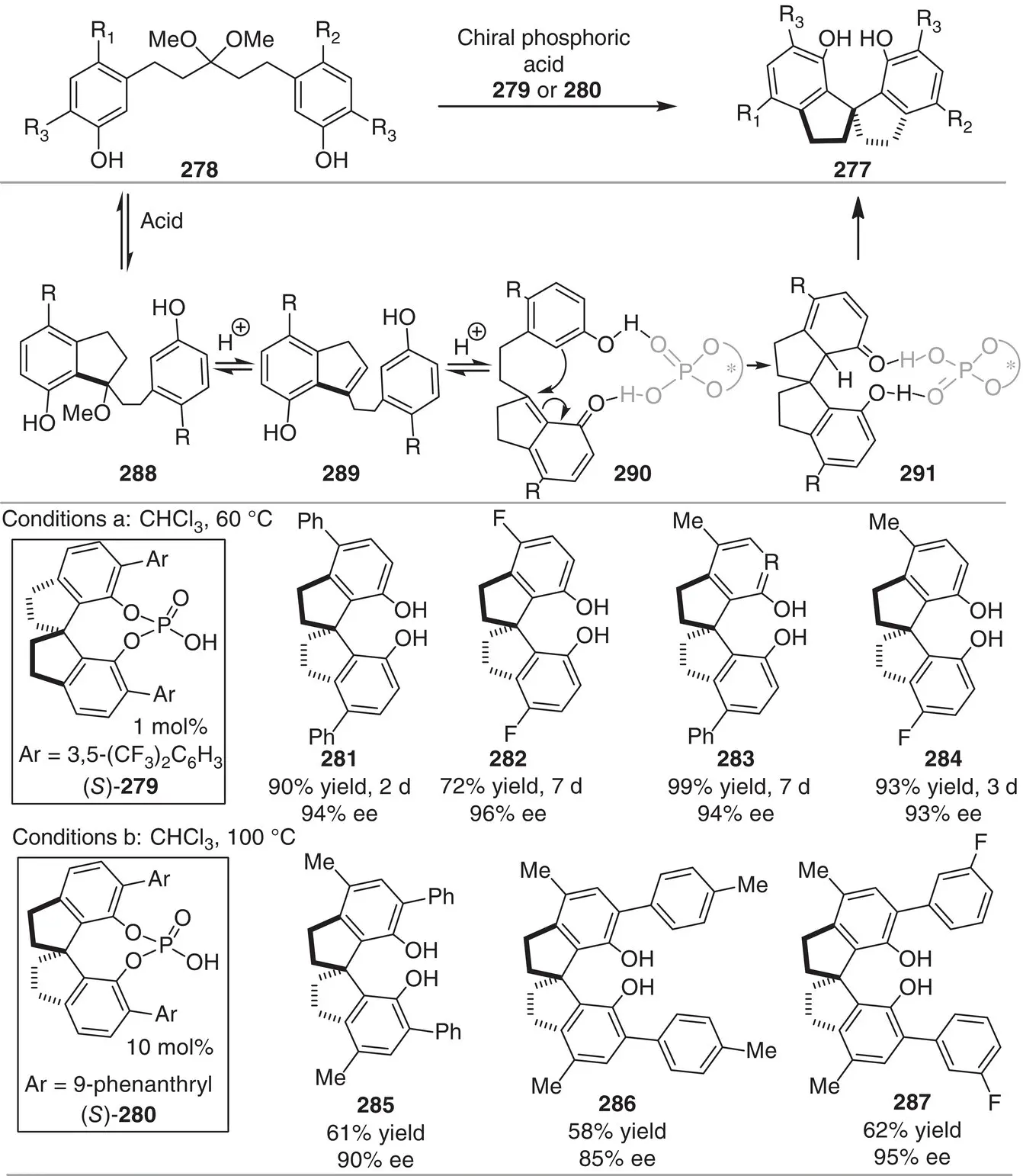
Scheme 3.25 Chiral phosphoric acid‐catalyzed asymmetric synthesis of SPINOL derivatives.
Source: Modified from Li et al. [37].
References
1 1 Baeyer, A. (1900). Ber. Dtsch. Chem. Ges. 33: 3771–3775.
2 2 (a) Lin, H. and Danishefsky, S.J. (2003). Angew. Chem. Int. Ed. 42: 36–51. (b) Galliford, C.V. and Scheidt, K.A. (2007). Angew. Chem. Int. Ed. 46: 8748. (c) Undheim, K. (2014). Synthesis 46: 1957–2006. (d) Smith, L.K. and Baxendale, I.R. (2015). Org. Biomol. Chem. 13: 9907–9933.
3 3 Zheng, Y., Tice, C.M., and Singh, S.B. (2014). Bioorg. Med. Chem. Lett. 24: 3673–3682.
4 4 For selected reviews, see: (a)Rios, R. (2012). Chem. Soc. Rev. 41: 1060–1074. (b) Xie, X., Huang, W., Peng, C., and Han, B. (2018). Adv. Synth. Catal. 360: 194–228. (c) Ding, A., Meazza, M., Guo, H. et al. (2018). Chem. Soc. Rev. 47: 5946–5996. (d) Xu, P.‐W., Yu, J.‐S., Chen, C. et al. (2019). ACS Catal. 9: 1820–1882.
5 5 For selected reviews, see: (a)Frühauf, H.‐W. (1997). Chem. Rev. 97: 523–526. (b) Reymond, S. and Cossy, J. (2008). Chem. Rev. 108: 5359–5406. (c) Pellissier, H. (2011). Adv. Synth. Catal. 353: 189–218. (d) Anand, A., Singh, P., Kumar, V., and Bhargava, G. (2019). RSC Adv. 9: 25554–25568.
6 6 For selected reviews, see: (a)Melchiorre, P., Marigo, M., Carlone, A., and Bartoli, G. (2008). Angew. Chem. Int. Ed. 47: 6138–6171. (b) Bertelsen, S. and Jørgensen, K.A. (2009). Chem. Commun.: 2178–2189. (c) Valero, G., Companyó, X., Bravo, N. et al. (2010). Synlett 13: 1883–1908. (d) Cherubini‐Celli, A., Mateos, J., Bonchio, M. et al. (2018). ChemSusChem 11: 3056–3070. (e) Silvi, M. and Melchiorre, P. (2018). Nature 554: 41–49.(f) Vega‐Peñaloza, A., Paria, S., Bonchio, M. et al. (2019). ACS Catal. 9: 6058–6072.
Читать дальшеИнтервал:
Закладка:
Похожие книги на «Spiro Compounds»
Представляем Вашему вниманию похожие книги на «Spiro Compounds» списком для выбора. Мы отобрали схожую по названию и смыслу литературу в надежде предоставить читателям больше вариантов отыскать новые, интересные, ещё непрочитанные произведения.
Обсуждение, отзывы о книге «Spiro Compounds» и просто собственные мнения читателей. Оставьте ваши комментарии, напишите, что Вы думаете о произведении, его смысле или главных героях. Укажите что конкретно понравилось, а что нет, и почему Вы так считаете.