In 2014, Franz and coworkers reported the asymmetric annulations of alkylidene oxindole 1and allyl silane 2for the synthesis of spirocyclic oxindole 3( Scheme 3.1) [8]. The reaction was conducted in the presence of ScCl 3and the chiral ( R , S )‐indapybox ligand 4and was proposed to proceed by the formation of β‐silyl carbocation 5through a Michael/1,2‐silyl shift/cyclization sequence.
Mechanistically, NaBArF played a dual role in the process, primarily is responsible to generating a cationic scandium complex and secondly is stabilizing the β‐silyl carbocation 5. Remarkably, this transformation represents the first example of a [3+2] annulation process involving allylsilanes and unsaturated carbonyl compounds to furnish chiral cyclopentanes in high yields (73–99%) and good to excellent stereocontrol (6 : 1–>20 : 1 dr and 64–99% ee).
Arai, Yamanaka, and coworkers presented in 2015 a [3+2] cycloaddition mediated by Cu(OTf) 2and the chiral PyBidine ligand 13for the synthesis of a variety of functionalized spiro[pyrrolidin‐3,3′‐oxindole]s 14( Scheme 3.2) [9]. The catalytic system, which was previously used for the endo‐selective [3+2] cycloaddition of iminoesters with nitroalkenes [10], furnished the spiranic products in excellent yields (83%–>99%) and high stereoselectivities (5 : 1–>20 : 1 and 91%–98% ee).
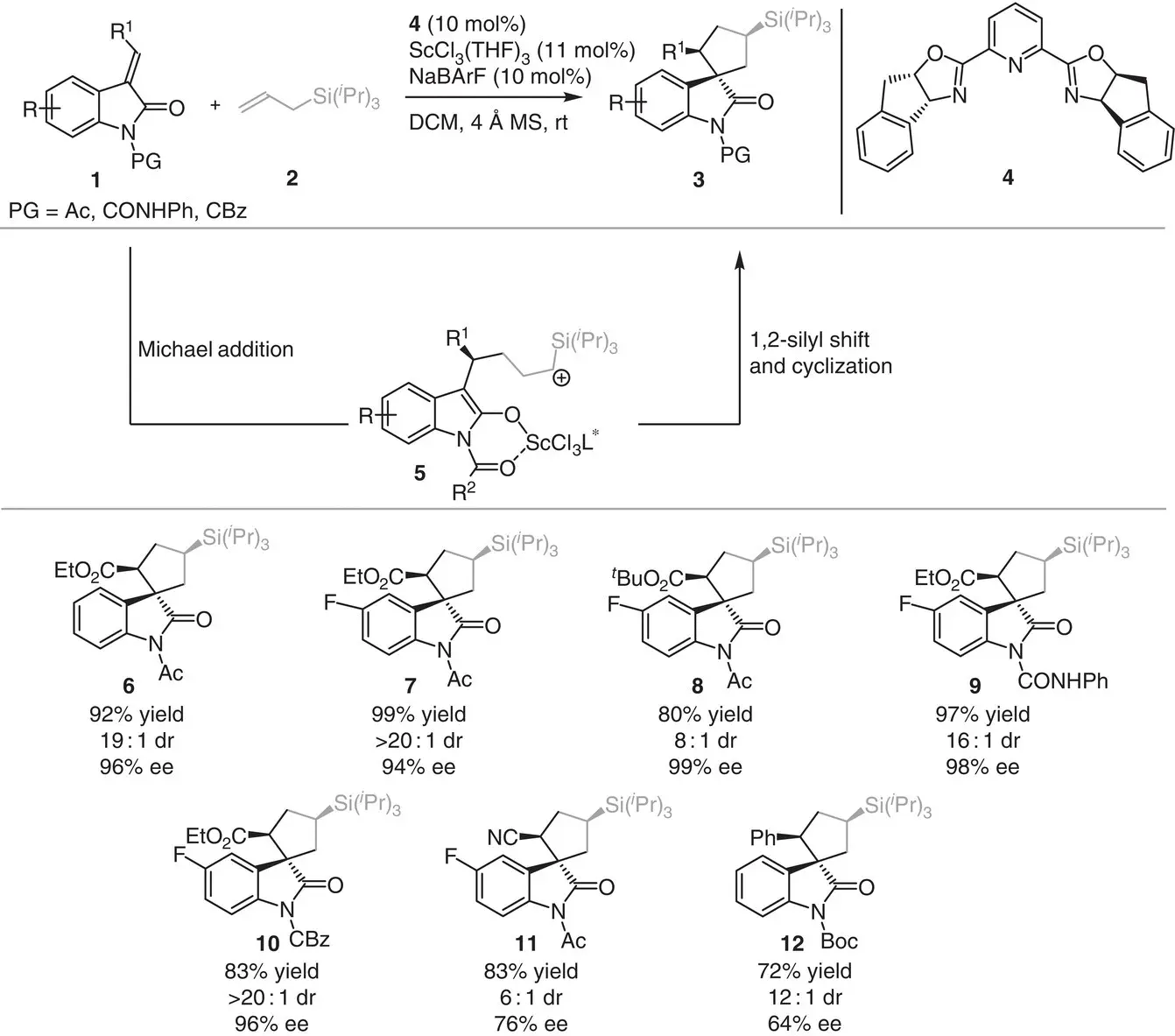
Scheme 3.1 Scandium‐catalyzed enantioselective carboannulation between alkylidene oxindole and allylsilanes.
Source: Modified from Ball‐Jones et al. [8].
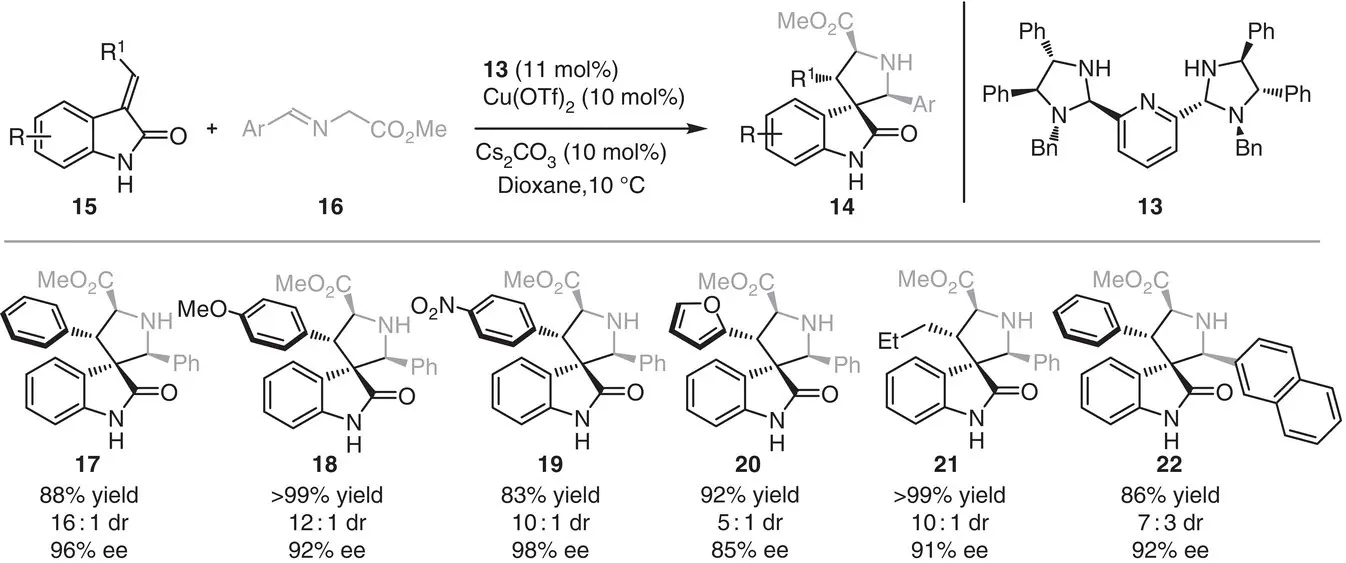
Scheme 3.2 Copper‐catalyzed asymmetric [3+2] cycloaddition between alkylidene oxindole and imino esters.
Source: Modified from Arai et al. [9].
In the same year, the group of Yu and Deng reported the first asymmetric 1,3‐dipolar cycloaddition of azomethine ylides 23with α‐alkylidene succinimides 24to obtain dispiropyrrolidine derivatives 25in good yields (up to 90%) and high stereoselectivities (>20 : 1 dr and 87–96% ee) ( Scheme 3.3) [11]. The methodology uses catalytic amounts of chiral N , O ‐ligand 26in the presence of Cu(OAc) 2to impart high stereocontrol over the [3+2] cyclization process. The catalytic system was also applied for the synthesis of pyrrolidinyl‐spirooxindole skeletons with excellent diastereo‐ and enantiocontrol.
Later on, the authors extended the application of their catalytic system (using a different ligand) to the 1,3‐dipolar cycloaddition of azomethine ylides to 5‐alkylidene thiazolidine‐2,4‐diones for the synthesis of spirocyclic pyrrolidine‐thiazolidinediones [12].
Shortly after, Feng, Liu, and coworkers reported that a combination of Mg(OTf) 2and the chiral N , N ′‐dioxide ligand 34is a highly effective catalytic system for the asymmetric [3+2] cycloaddition of methyleneindolinones 1with N , N ′‐cyclic azomethine imines 35to produce pyrazolidine‐based spirooxindoles 36[13]. This strategy allows the construction of valuable contiguous tertiary and quaternary stereocenters ( Scheme 3.4).
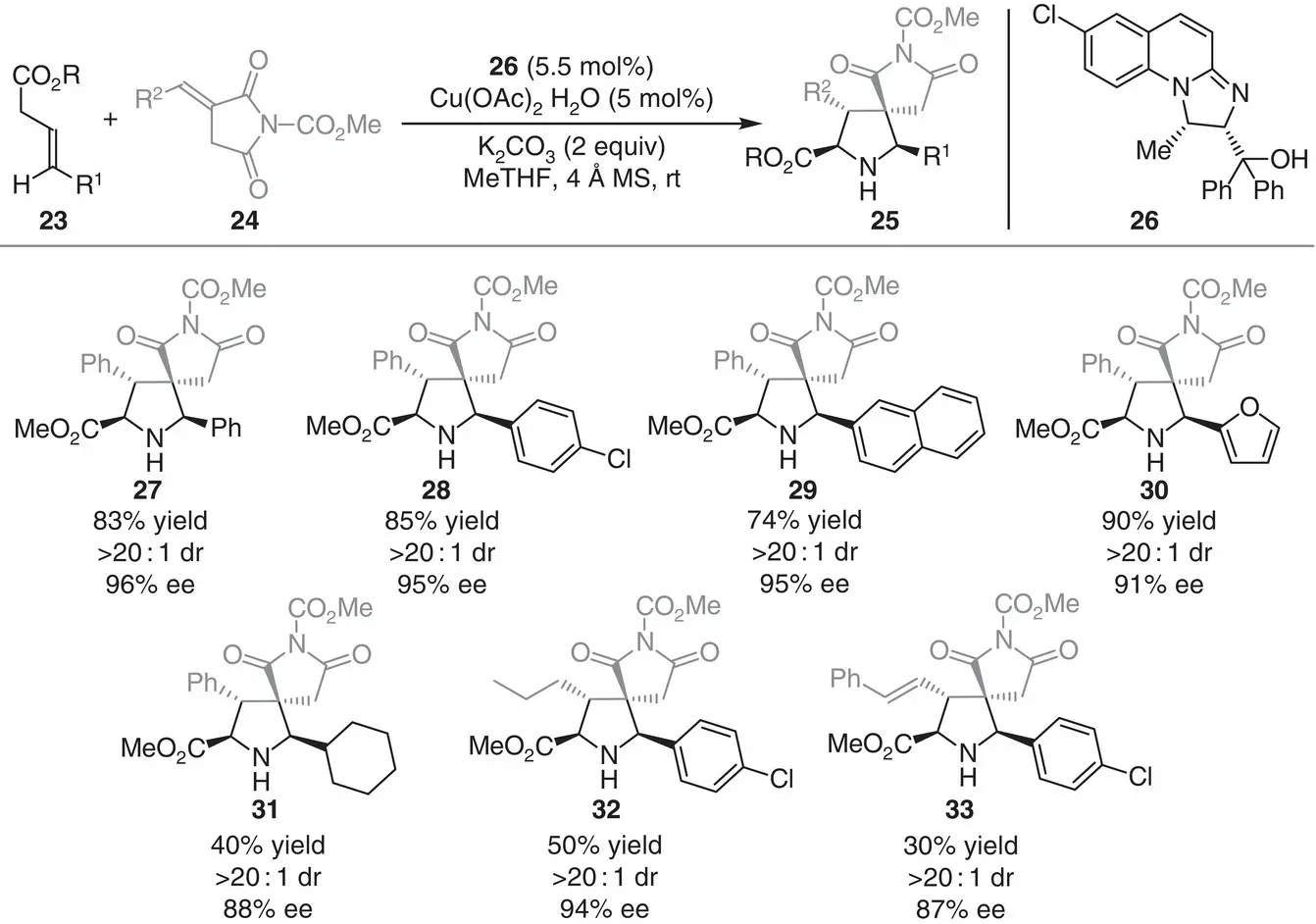
Scheme 3.3 Copper‐catalyzed asymmetric construction of dispiropyrrolidine skeletons via 1,3‐dipolar cycloaddition between azomethine ylides and α‐alkylidene succinimides.
Source: Modified from Yang et al. [11].
A weak positive nonlinear effect was observed between the enantiomeric excess of the product 36and the chiral ligand 34. Hence, the authors suggest the presence of both monomeric and oligomeric catalytic species in the reaction medium, with the monomeric complex being more active. Subsequently, an asymmetric [3+2] cycloaddition of methyleneindolinones with nitrones was also reported by the same authors using the combination of chiral N , N ′‐dioxide ligand and Co(BF 4) 2·H 2O to synthesize spirooxindole derivatives in good yield and excellent diastereo‐ and enantiocontrol [14].
In 2016, Cramer and coworkers reported the [3+2] annulation between alkynes 47and cyclic sulfonimines 46catalyzed by a chiral Rh complex for the synthesis of spirocyclic sultams 44( Scheme 3.5) [15]. The methodology involves the use of a chiral cyclopentadienyl ligand 45. Under the optimized reaction conditions, the enantioenriched sultams are obtained in good to excellent yields (50–99%) and enantioselectivites (75–90%). The proposed catalytic cycle is initiated by the C–H activation of the sulfonimine 46, which is directed by the N ‐sulfonyl imino group, to generate the rhodacycle 48. Alkyne migratory insertion of intermediate 48followed by the addition to the imine bond generates the spiro compound 44. Low regioselectivities were observed in the case of unsymmetrical aryl alkynes 47. This is the major limitation of this process.
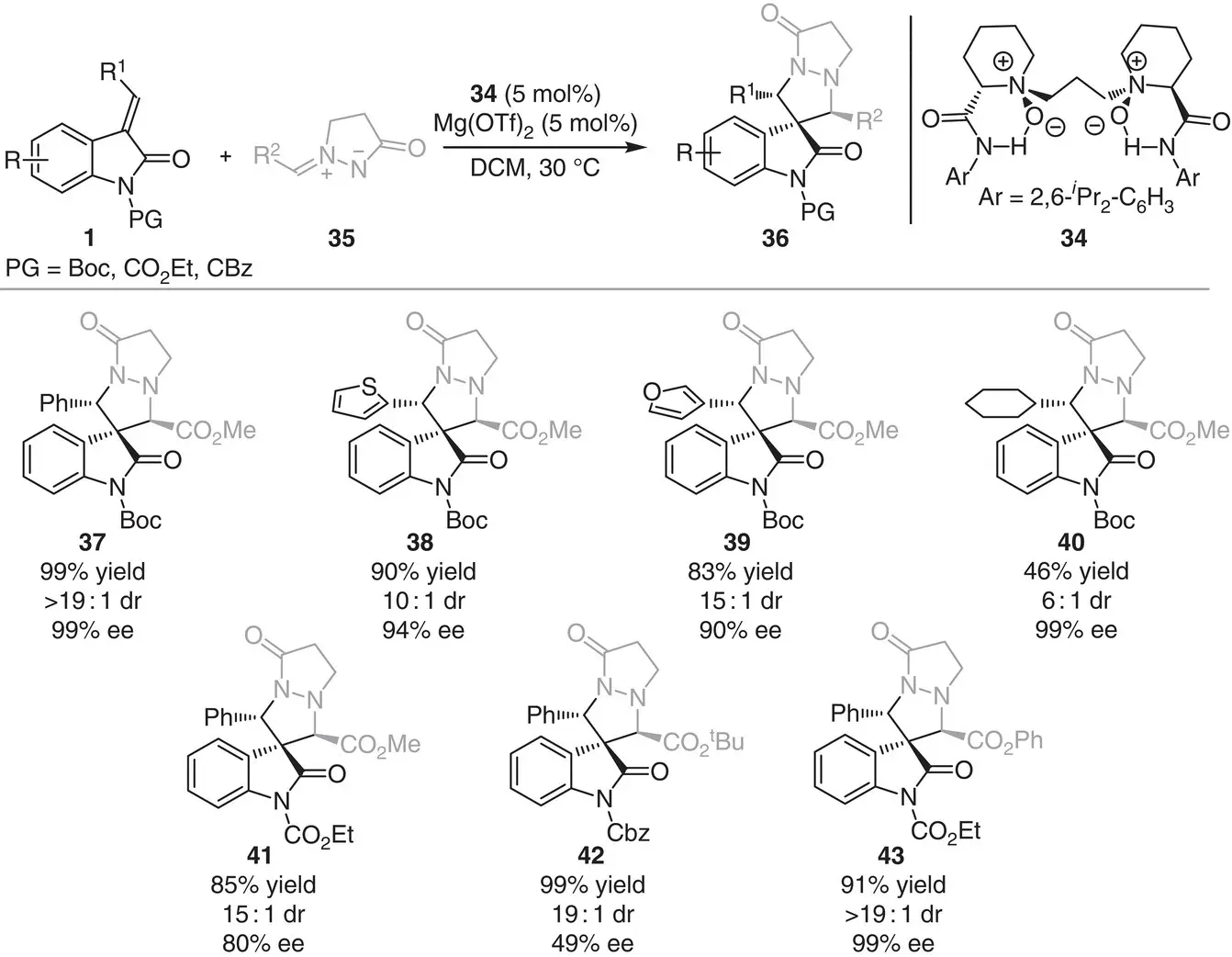
Scheme 3.4 Magnesium‐catalyzed asymmetric [3+2] cycloaddition between methyleneindolinones and N , N ′‐cyclic azomethine imines.
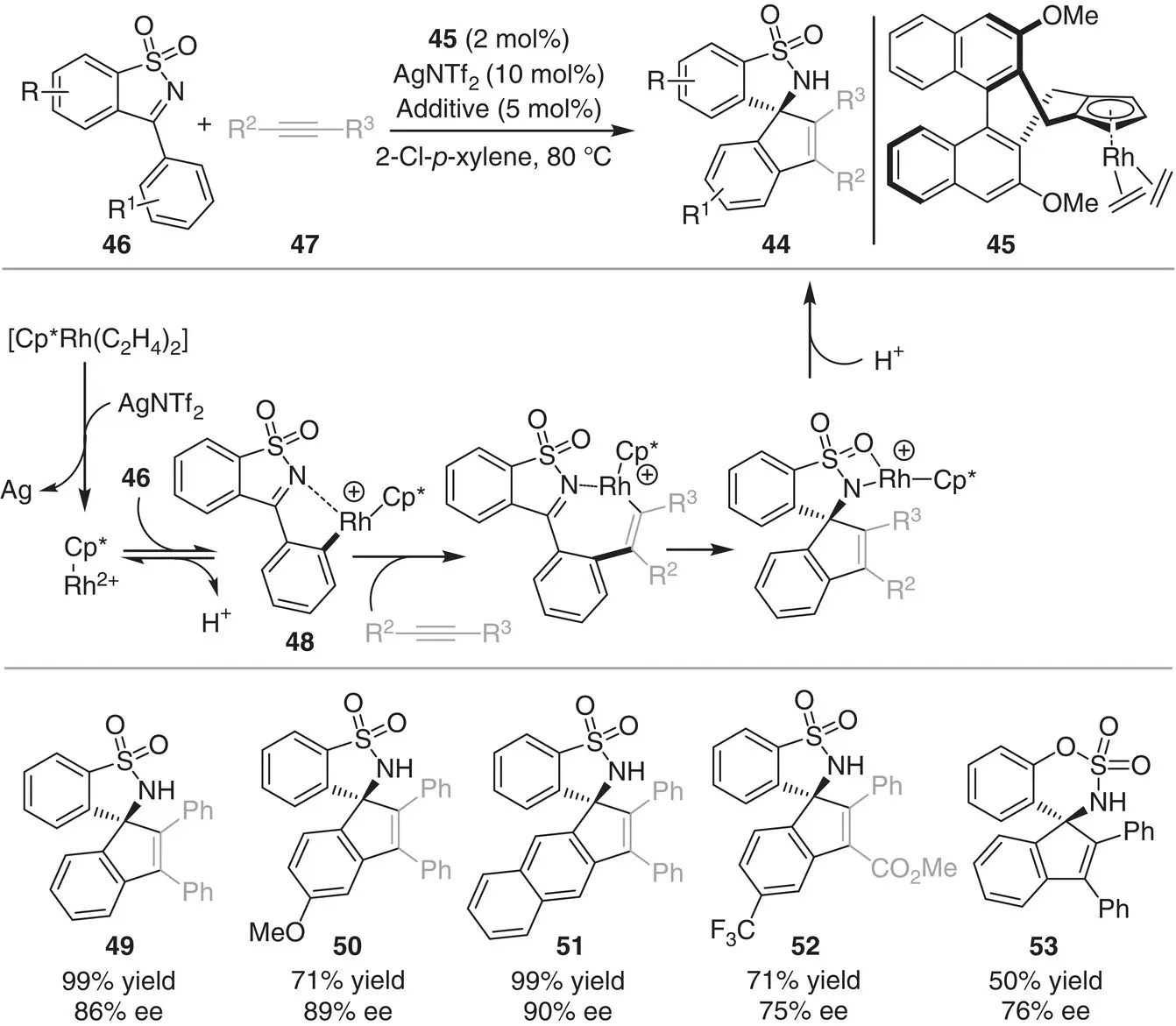
Scheme 3.5 Rhodium‐catalyzed enantioselective [3+2] annulation to form spirocyclic sultams.
Source: Modified from Pham and Cramer [15].
In the same year, the group of Zhao reported the asymmetric [3+2] cycloaddition of p ‐quinone methides 54with vinyl cyclopronanes 55and epoxides 56( Scheme 3.6) [16]. The reaction proceeds through a 1,6‐conjugate addition of Pd‐activated vinyl cyclopronanes 55and epoxides 56to p ‐quinone methides 54followed by the annulation event. Ferrocene‐based chiral phosphine 57was the ligand of choice for the annulations of epoxides, while chiral spiro phosphene 58was found to be optimal in the case of vinyl cyclopropanes, obtaining in both the cases the products 61‐65in good to high yields (71–98%) and excellent stereocontrol (3 : 1–>20 : 1 dr and 51–99% ee).
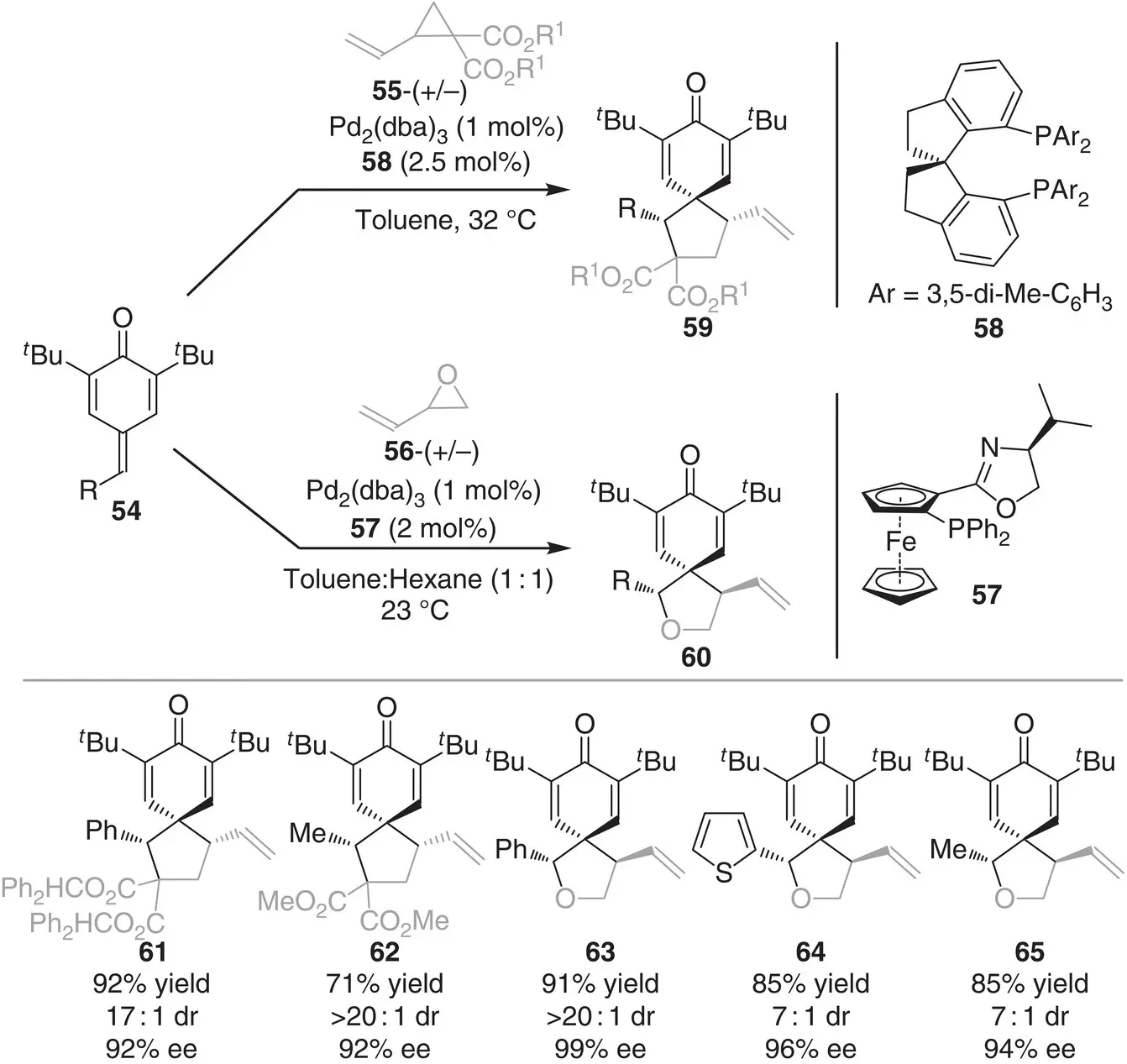
Scheme 3.6 Palladium‐catalyzed stereoselective 1,6‐conjugate addition/annulation of vinyl epoxides/cyclopropanes to p ‐quinone methides.
Source: Modified from Ma et al. [16].
Читать дальше