In 2018, the group of Enders established the first Cu‐catalyzed enantioselective Kinugasa/Michael domino reaction for the synthesis of spirocyclic β‐lactams 66by desymmetrization of prochiral cyclohexadienone 67with nitrones 68( Scheme 3.7) [17]. Cu(OTf) 2and indane‐BOX ligand 69was found to be the optimal catalytic system for the reaction. Mechanistically, the reaction starts with the formation of Cu‐acetylide intermediate 70which underwent [3+2] cycloaddition with nitrones 68to form Cu‐bound isoxazoline intermediate 71. A rearrangement of the intermediate 71affords the tethered four‐membered copper enolate intermediate 72, which subsequently participates in desymmetric Michael addition to afford the spirocyclic β‐lactams 66in a highly enantio‐ and diastereoselective fashion (>20 : 1 dr and 82–96% ee).
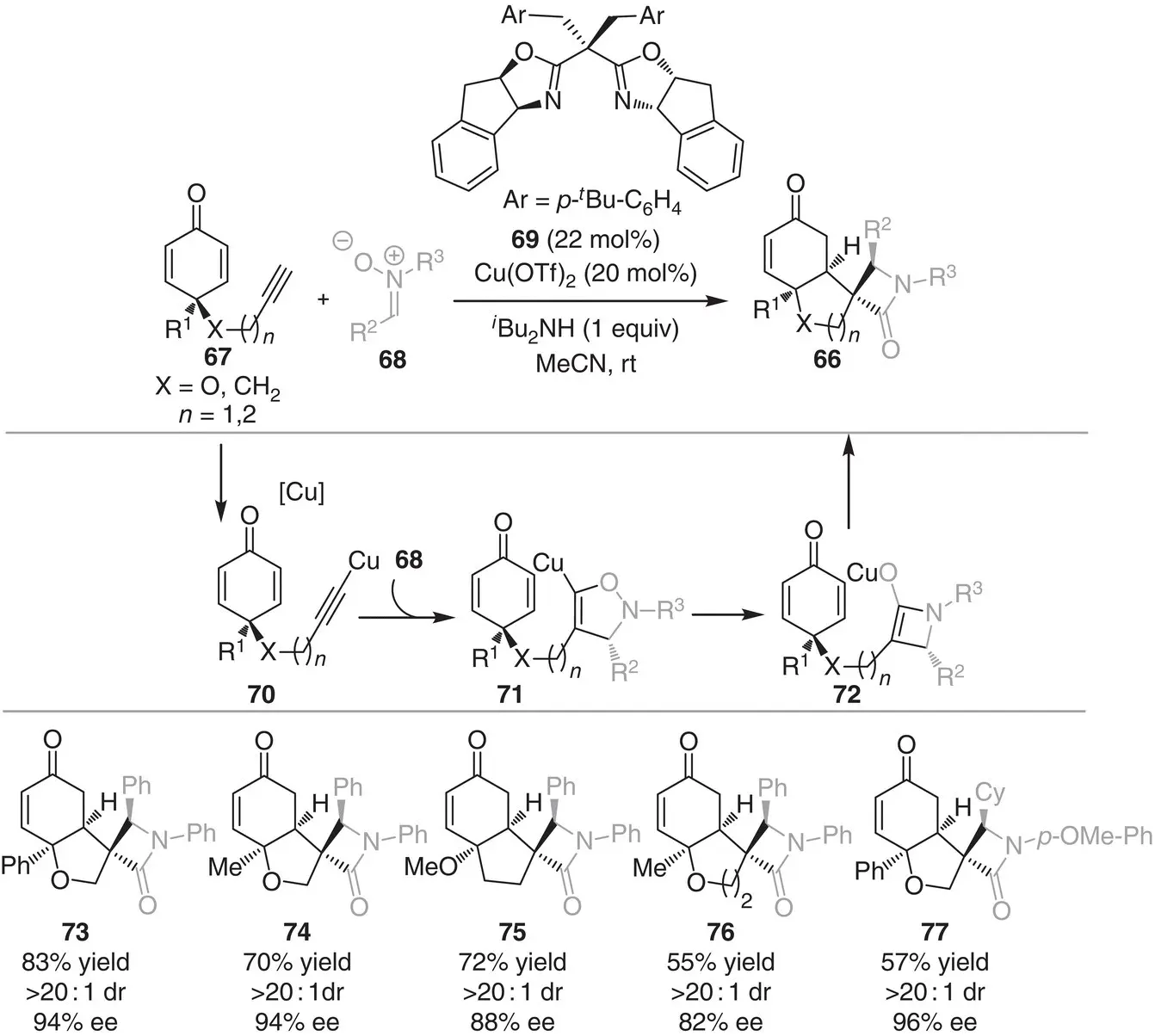
Scheme 3.7 Copper‐catalyzed asymmetric synthesis of spirocyclic β‐lactams through Kinugasa/Michael domino sequence.
Source: Modified from Shu et al. [17].
In 2018, Waldmann and Antonchick reported the enantioselective synthesis of spirotropanyl oxindoles 78by a bimetallic relay strategy ( Scheme 3.8) [18]. Transient azomethine ylides 79were generated from E ‐oximino α‐diazo ketones 80in the presence of an achiral Rh(II) complex, which subsequently underwent intermolecular [3+2] cycloaddition with 3‐alkenyloxindole 81catalyzed by the chiral N , N ‐dioxide Nd(III) Lewis acid complex. The products are obtained in high yields (54–97%) with high enantio‐ and diastereoselectivity (3.2 : 1 dr and 77%–>99% ee).
3.2.2 Organometallic [4+2] Cycloaddition Strategies to Construct Spiro Compounds
In 2015, Liu, Feng, and coworkers reported a regio‐ and enantioselective aza‐Diels–Alder reaction of 3‐vinylindoles 90with isatin‐derived ketimines 91( Scheme 3.9) [19]. The reaction uses Ni(II) complex together with the chiral N , N ′‐dioxide ligand 92, providing a wide range of spiroindolones 93in good to high yields (75–96%), high enantioselectivities (62–94% ee), and complete diastereocontrol. Mechanistically, the reaction commenced by the activation of isatin‐derived ketimines 91by the chiral L‐PiPr 2/Ni(II) complex through coordination with the two carbonyl groups, via a lowering LUMO strategy. The 2,4,6‐triisopropylaniline moieties in the chiral N , N ′‐dioxide ligand 92ensured the Si ‐face approach of the 3‐vinylindoles 90, providing the product exclusively in exo fashion.
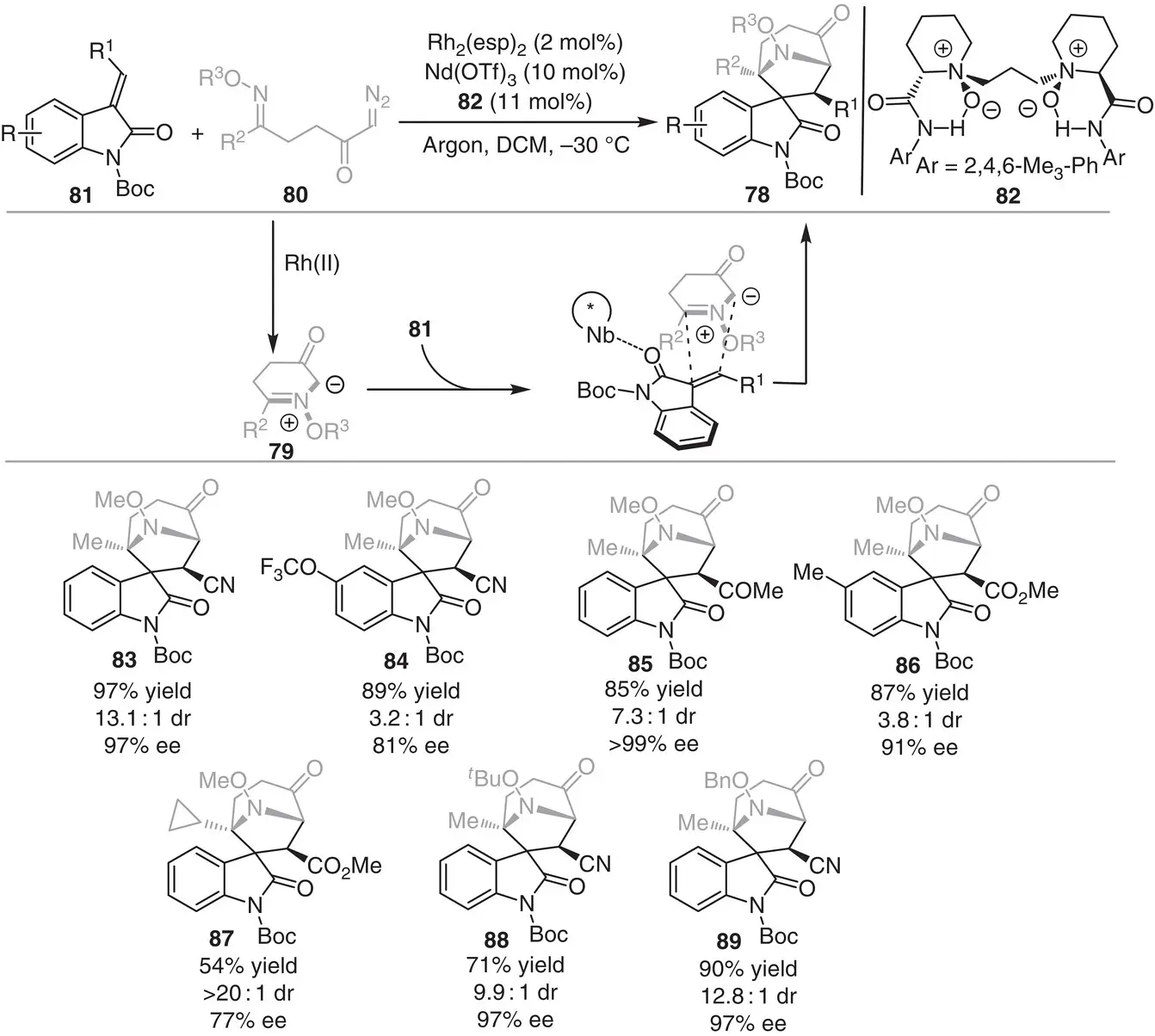
Scheme 3.8 Bimetallic relay catalysis for the enantioselective synthesis of the spirotropanyl oxindole.
Source: Modified from Jia et al. [18].
Recently, the same group established the asymmetric Diels–Alder reaction/[3,3]sigmatropic rearrangement cascade of methyleneindolinones 1with 1‐thiocyanatobutadienes 101for the synthesis of spiranic cyclohexenyl isothiocyanates 102( Scheme 3.10) [20]. The reaction uses Ni(II) complex and the chiral N , N ′‐dioxide ligand 103, providing a range of spiro cyclohexenyl isothiocyanates in high yields (64–97%) with excellent diastereo (>15 : 1 in all the cases) and enantioselectivities (65–94% ee). Mechanistically, the reaction involves the initial activation of methyleneindolinones 1by the chiral L‐PiPr 2/Ni(II) complex through coordination with the two carbonyl groups, generating intermediate 104. Subsequently, the 1‐thiocyanatobutadienes 101participates in a stereoselective quasi‐concerted Diels–Alder cycloaddition from the Re face of 104to afford intermediate 105, which further undergoes a suprafacial [3,3]sigmatropic rearrangement to furnish the final product 102. Interestingly, the authors applied the developed method to the gram‐scale synthesis of NITD609, an antimalarial active spirocyclic indole. Shortly after, the same group also developed a catalytic asymmetric Diels–Alder reaction for the synthesis of optically pure spiro[cyclohexane‐oxindoline] derivatives using Zn(OTf) 2and a chiral N , N ′‐dioxide ligand [21].
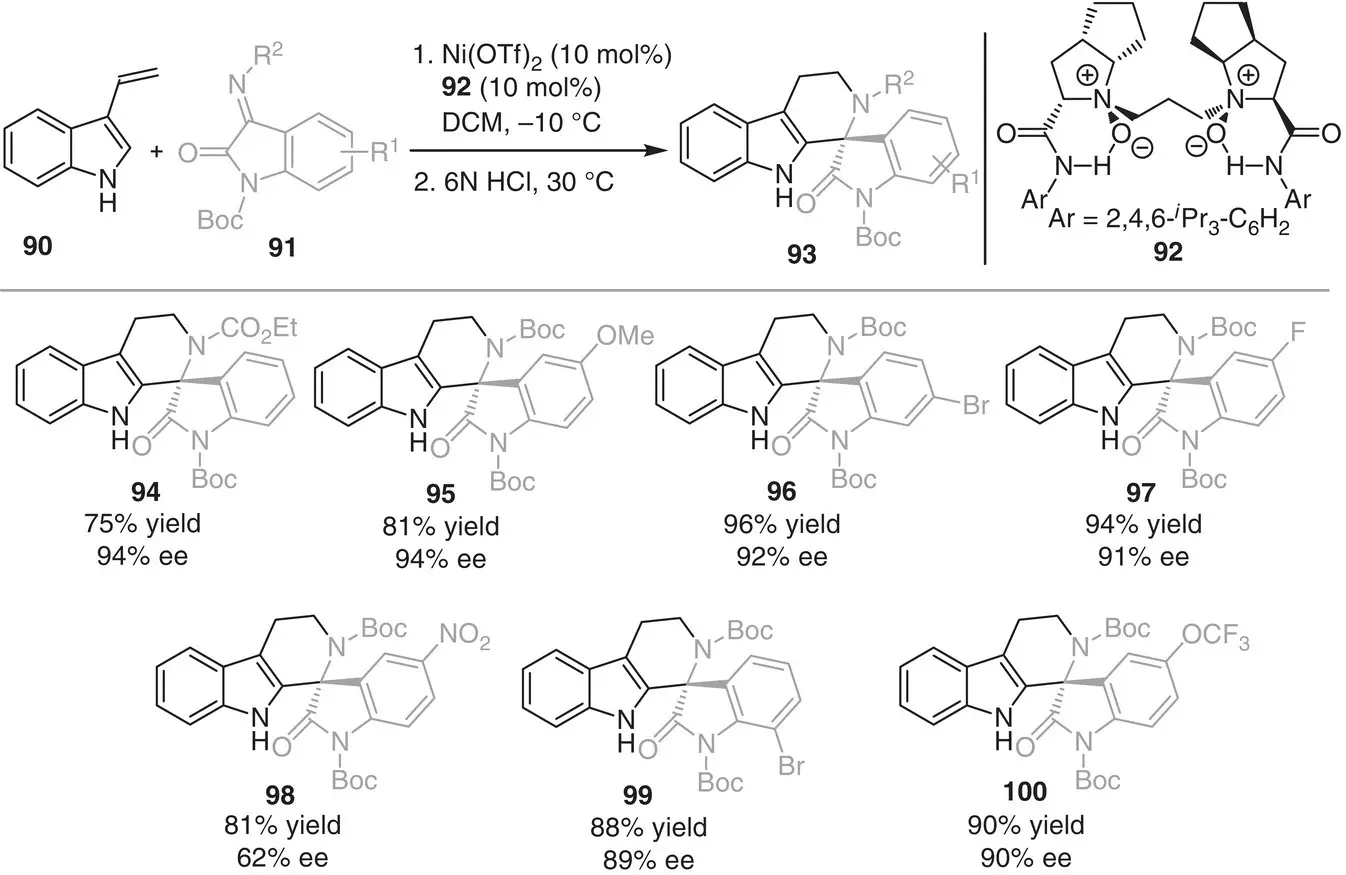
Scheme 3.9 Regio‐ and enantioselective aza‐Diels–Alder reactions of 3‐vinylindoles.
Source: Modified from Zheng et al. [19].
3.2.3 Organometallic Miscellaneous Strategies to Construct Spiro Compounds
The following reports are based on the construction of strained small ring spirocyclic scaffolds and on the utilization of merged metal‐ and organocatalytic strategies. The first example deals with an enantioselective [2+2] cycloaddition of disubstituted ketenes 114and isatins 115for the synthesis of spirocyclic β‐lactones 116, reported by the group of Feng in 2014 ( Scheme 3.11) [22]. The method utilizes Sc(OTf) 3as Lewis acid catalyst in the presence of the chiral N , N ′‐dioxide ligand 117, responsible for controlling the stereochemistry of the product 116. A variety of chiral β‐lactones were obtained in excellent yield (80–96%) with very high diastereo‐ and enantioselectivity (>20 : 1 dr and 88–97% ee).
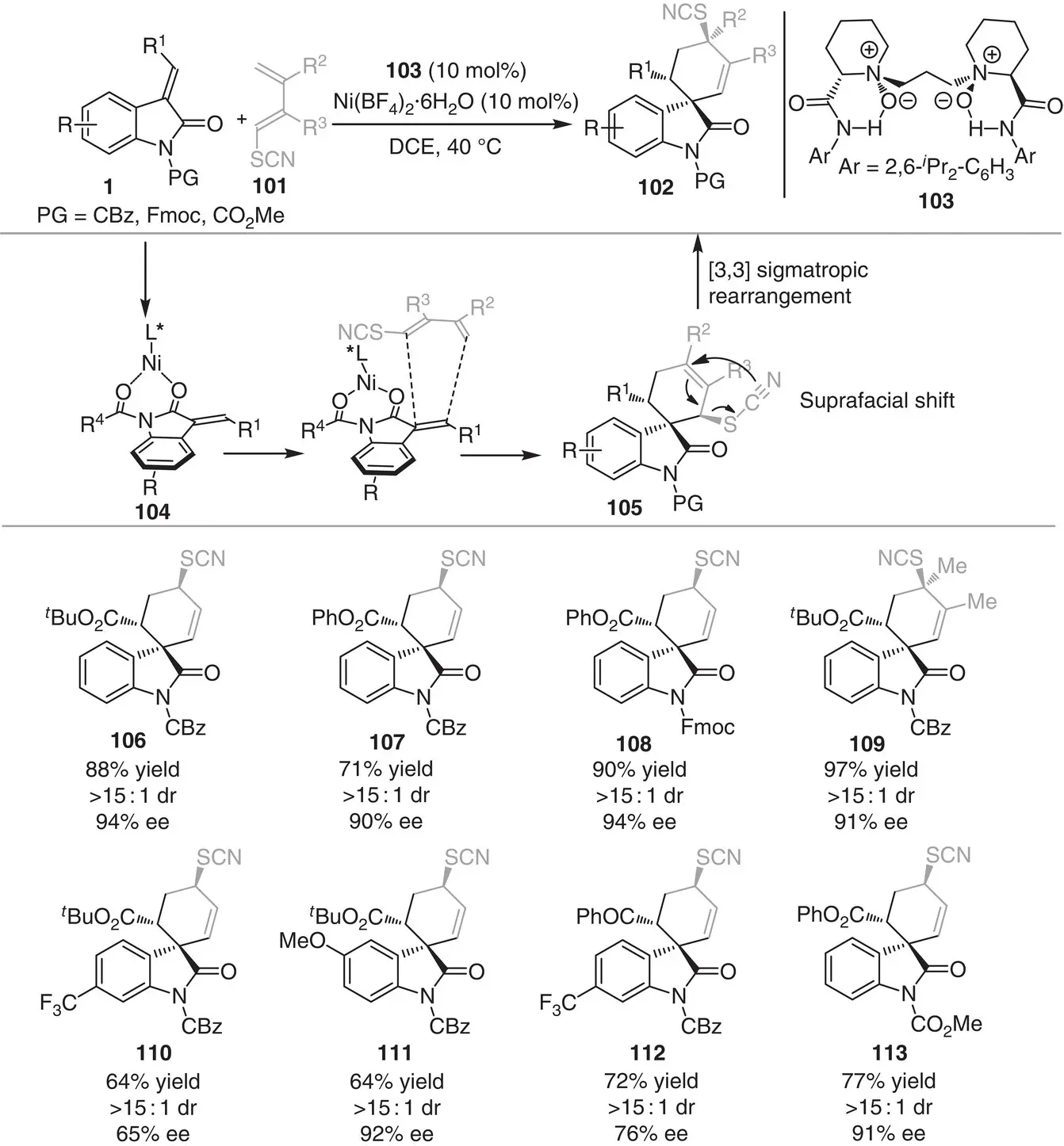
Scheme 3.10 Ni‐catalyzed asymmetric Diels–Alder/[3,3]sigmatropic rearrangement cascade between methyleneindolinones with 1‐thiocyanatobutadienes.
Source: Modified from Zhou et al. [20].
In 2015, Tanaka and coworkers outlined an enantioselective [2+2+2] cycloaddition approach to construct spiro‐cyclohexadienes 123, starting from terminal alkynes 124, acetylenedicarboxylates 125, and cyclopropylideneacetamides 126( Scheme 3.12) [23].
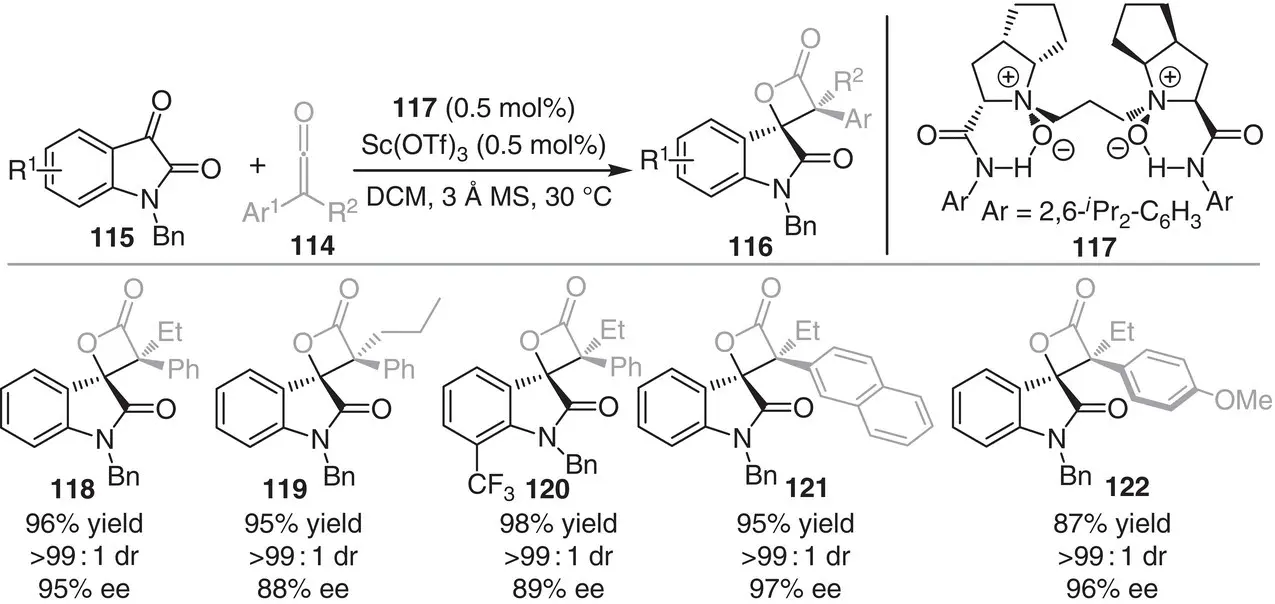
Scheme 3.11 Scandium‐catalyzed asymmetric cycloaddition between ketenes and isatins.
Source: Modified from Hao et al. [22].
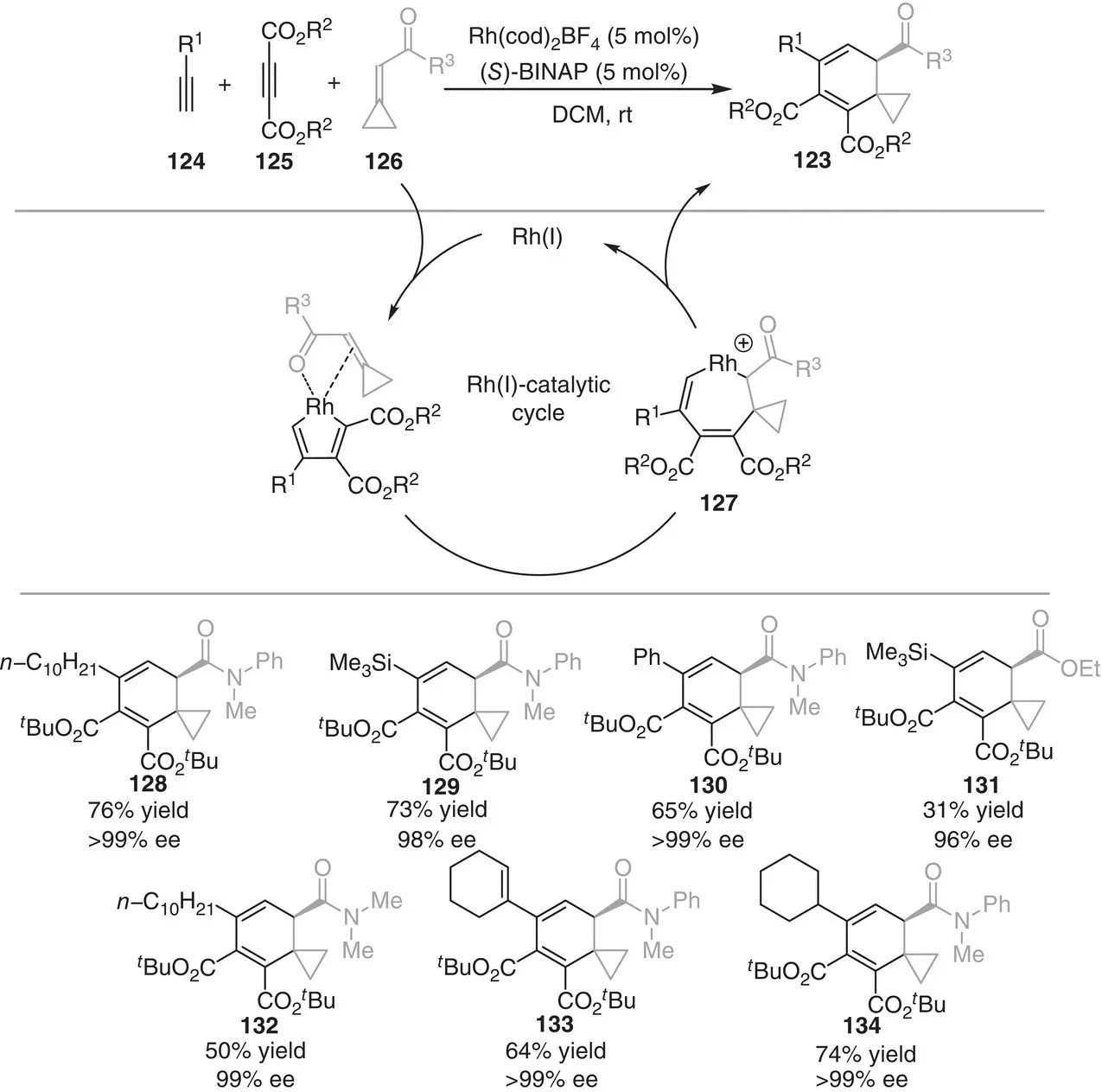
Scheme 3.12 Rhodium‐catalyzed [2+2+2] cycloadditions between alkynes and cyclopropylideneacetamides.
Source: Based on Yoshida et al. [23].
Читать дальше