Spiro Compounds
Здесь есть возможность читать онлайн «Spiro Compounds» — ознакомительный отрывок электронной книги совершенно бесплатно, а после прочтения отрывка купить полную версию. В некоторых случаях можно слушать аудио, скачать через торрент в формате fb2 и присутствует краткое содержание. Жанр: unrecognised, на английском языке. Описание произведения, (предисловие) а так же отзывы посетителей доступны на портале библиотеки ЛибКат.
- Название:Spiro Compounds
- Автор:
- Жанр:
- Год:неизвестен
- ISBN:нет данных
- Рейтинг книги:3 / 5. Голосов: 1
-
Избранное:Добавить в избранное
- Отзывы:
-
Ваша оценка:
- 60
- 1
- 2
- 3
- 4
- 5
Spiro Compounds: краткое содержание, описание и аннотация
Предлагаем к чтению аннотацию, описание, краткое содержание или предисловие (зависит от того, что написал сам автор книги «Spiro Compounds»). Если вы не нашли необходимую информацию о книге — напишите в комментариях, мы постараемся отыскать её.
A comprehensive treatment of the latest research in, and applications of, spiro compounds Spiro Compounds: Synthesis and Applications
Spiro Compounds
Spiro Compounds — читать онлайн ознакомительный отрывок
Ниже представлен текст книги, разбитый по страницам. Система сохранения места последней прочитанной страницы, позволяет с удобством читать онлайн бесплатно книгу «Spiro Compounds», без необходимости каждый раз заново искать на чём Вы остановились. Поставьте закладку, и сможете в любой момент перейти на страницу, на которой закончили чтение.
Интервал:
Закладка:
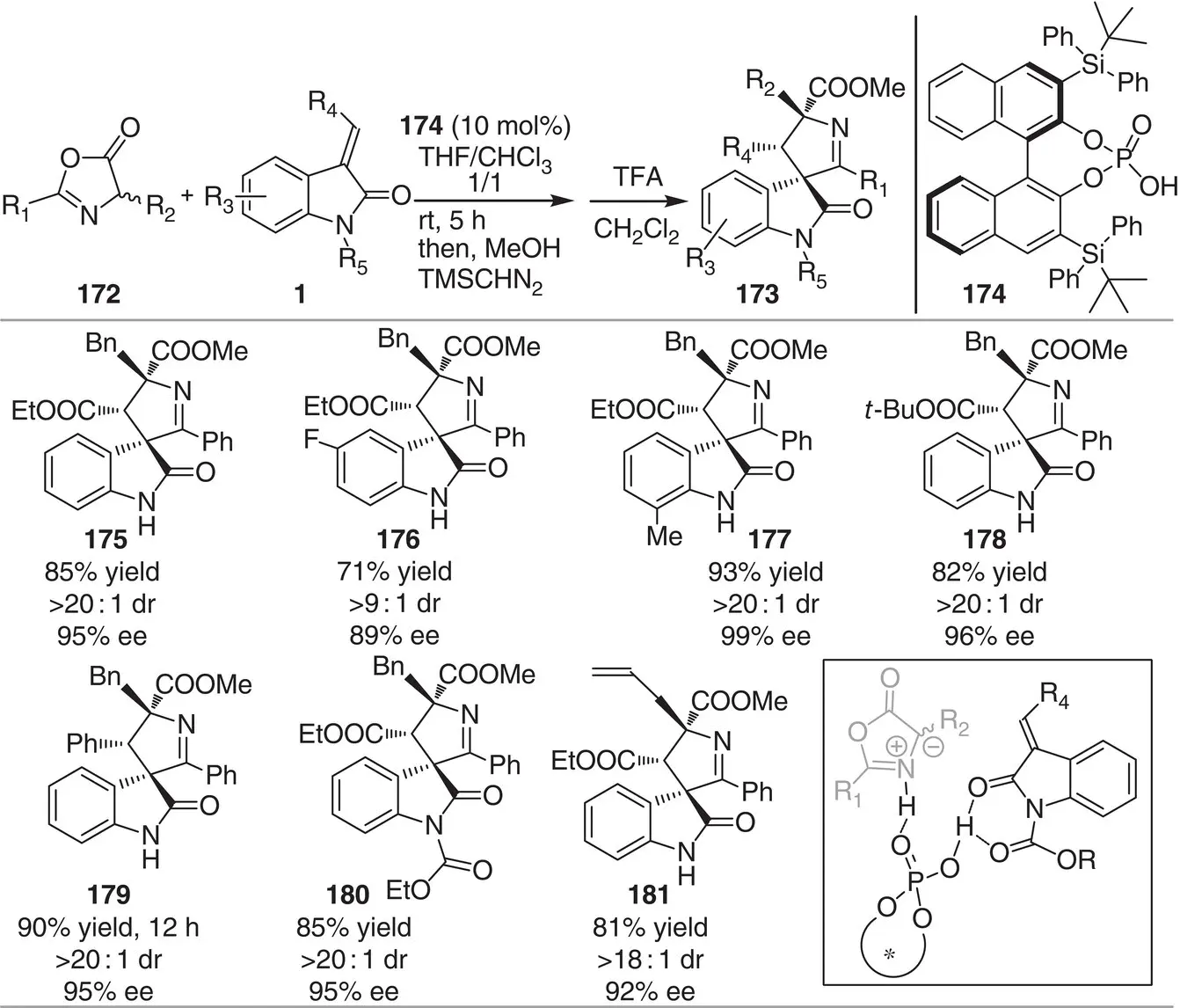
Scheme 3.16 Chiral phosphoric acid‐catalyzed enantioselective 1,3‐dipolar cycloaddition between methyleneindolinones and azlactones.
Source: Modified from Zhang et al. [28].
3.3.2 Organocatalytic [4+2] Cycloaddition Strategies to Construct Spiro Compounds
In 2016, Jørgensen and coworkers reported the asymmetric synthesis of spiroindenes 182containing up to four contiguous stereocenters via trienamine catalysis ( Scheme 3.17) [29]. The reaction between benzofulvenes 183and 2,4‐dienals 184catalyzed by the diphenylprolinol silyl ether catalyst 138and ortho‐fluorobenzoic acid ( o ‐FBA) as additive furnish the indenes spiro‐fused to cyclohexene compounds 182through a formal [4+2] cycloaddition. A range of differently substituted spiroindene compounds was formed efficiently in high yields (66–98%) and excellent stereoselectivities (6 : 1–>20 : 1 dr and 79–99% ee). The authors proposed that the all‐ E ‐configured trienamine intermediate reacts with the benzofulvene via an endo transition state where charge stabilization between the nitrile group and the enamine moiety could be a significant factor for the catalytic performance and the observed stereoselectivity. Finally, the Michael acceptor present in products 182was demonstrated to facilitate further intramolecular cyclizations to yield complex polycyclic products. Thus, reduction of the aldehyde moiety to the alcohol or reductive amination with benzylamine triggered the intramolecular oxa‐ or aza‐Michael additions to yield the tetracyclic spirocompounds 192and 193as a single diastereoisomer in 58 and 54% yield, respectively.
The same group reported the first asymmetric organocatalyzed [4+2]‐cycloaddition reaction via a tetraenamine intermediate 194a–bfor the construction of highly functionalized spirocyclic oxindoles 195( Scheme 3.18) [30]. The reaction between 2‐(cyclohepta‐1,3,5‐trien‐1‐yl)acetaldehyde 196and 3‐alkyliden oxindoles 197in the presence of diphenylprolinol silyl ether catalyst 198forms a wide range of spiranic products in high stereoselectivities (86 : 14–>95 : 5 dr and 68–95% ee) containing a highly functionalized six‐membered ring with an annulated seven‐membered ring. Mechanistic investigations suggested a stepwise mechanism via tetraenamine formation, conjugate addition, catalyst hydrolysis, and a final cyclization/isomerization sequence. Although the s‐trans conformer of the nucleophilic enamine 194awas detected by NMR spectroscopy, the authors proposed that the reactive intermediate is the enamine 194b, based on computational studies as well as the stereochemical outcome of the reaction.
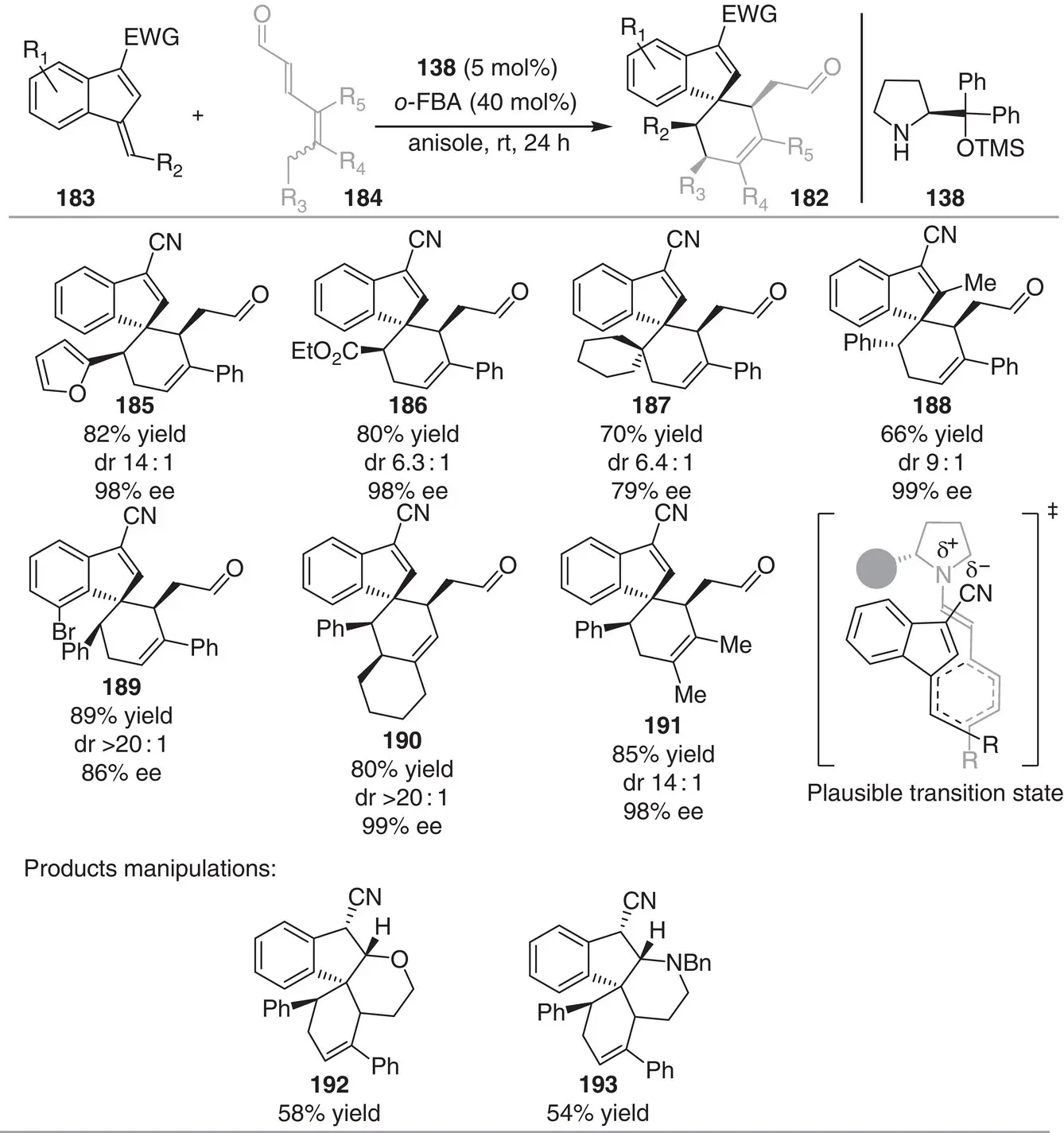
Scheme 3.17 Stereoselective synthesis of spiroindene via trienamine catalysis with benzofulvenes.
Source: Modified from Donslund et al. [29].
In 2014, Connon and coworkers developed the first catalytic asymmetric Tamura [4+2] cycloaddition reaction for the construction of spiro compounds 206( Scheme 3.19) [31]. Alkylidene oxindoles 203react with enolizable anhydrides 204by means of a quinine‐derived squaramide bifunctional catalyst 205to generate the densely functionalized spirooxindoles 206bearing three contiguous stereocenters with excellent enantiocontrol (89%–>99% ee). The methodology tolerates a wide range of enolizable anhydrides and alkylidene oxindoles with different stereoelectronic requirements. Interestingly, using 20 mol% of the organocatalyst at −50 °C, the kinetic diastereomer 213can be isolated in 98% ee. Thus, by simply changing the reaction temperature is possible to switch the stereoselectivity of the process, an important approach in target‐oriented synthesis.
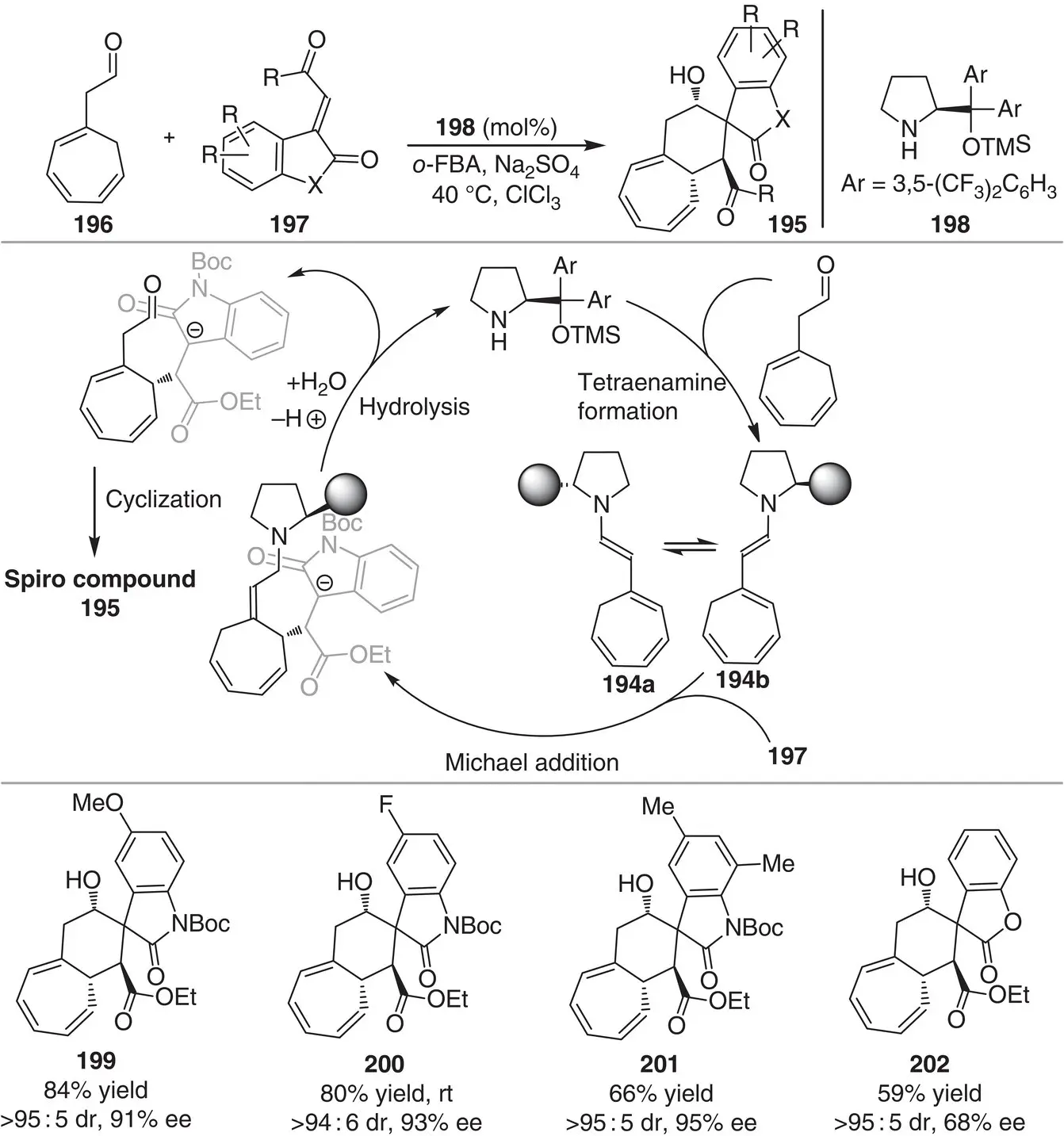
Scheme 3.18 Organocatalytic [4+2] addition reactions via tetraenamine intermediate.
Source: Modified from Stiller et al. [30].
In 2017, Yang and coworkers designed a sulphonamide‐based H‐bond donor organocatalyst derived from L ‐pyroglutamic acid, a cheap chiral feedstock available in both enantiomeric forms ( Scheme 3.20) [32]. The catalyst 214was successfully implemented in the [4+2] cyclization between methyleneindolinones 15and 2‐vinyl‐1H‐indoles 215to assemble carbazolespirooxindoles 216in excellent yields (62–99%) and stereoselectivities (81–>99% ee, >20 : 1 dr). The methodology has a broad tolerance to structural changes in both substrates (25 examples). Control experiments suggest that N−H groups in both substrates actively interact with the organocatalyst 214, having sizable influence on the observed enantioselectivity ( Scheme 3.20). Moreover, water plays a critical role in the enantioselectivity via an H 2O‐relay H‐bond between the catalyst and the 2‐vinyl‐1H‐indole.
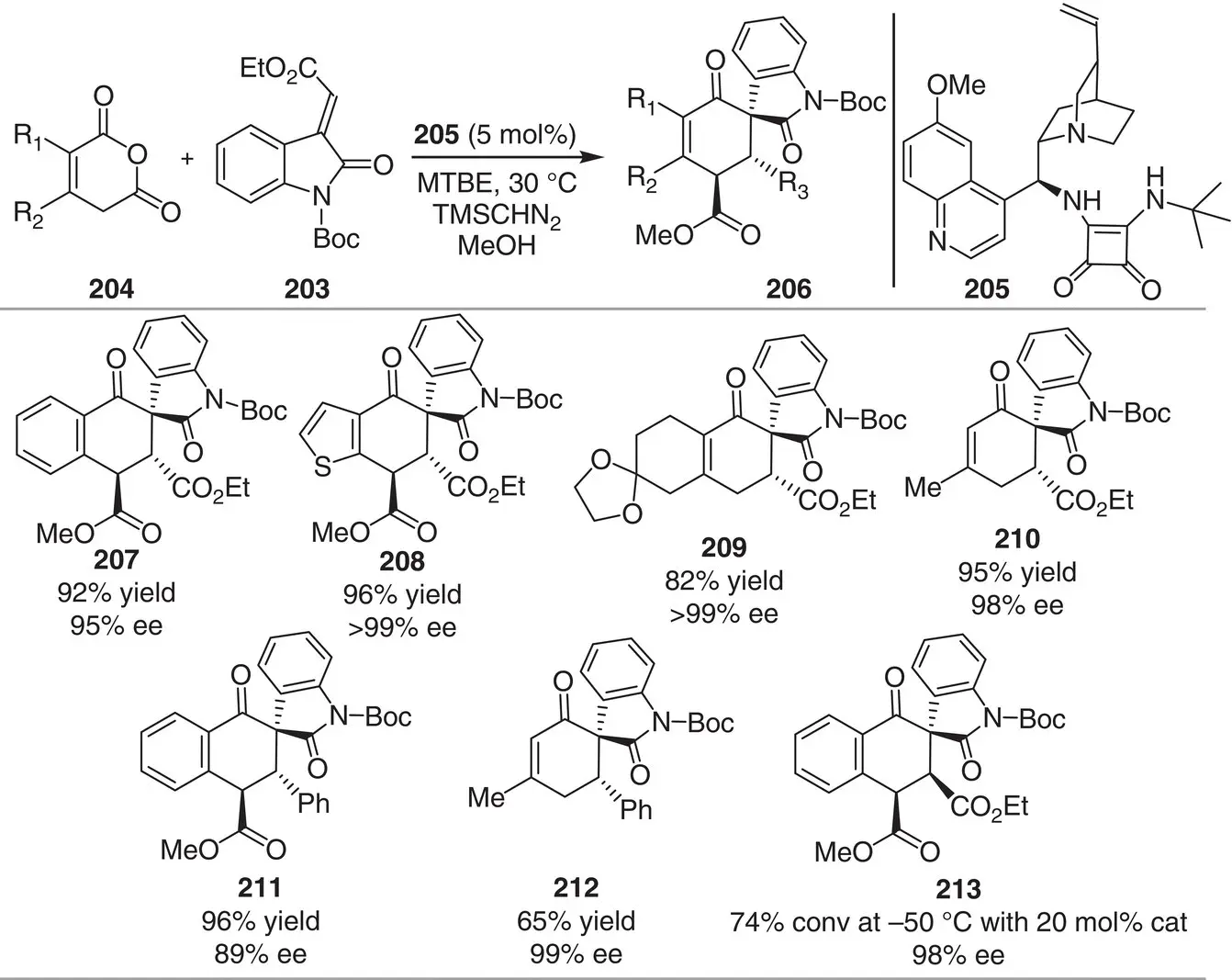
Scheme 3.19 Chiral H‐bond donor‐catalyzed asymmetric Tamura [4+2]‐cycloaddition.
Source: Modified from Manoni and Connon [31].
3.3.3 Organocatalytic [4+3] and [2+2] Cycloaddition and Switchable Strategies to Construct Spirocompounds
Very recently, the group of Fu and Huang developed an NHC‐enabled formal [4+3] annulation of flexible oxotryptamines 223with enals 224under mild reaction conditions ( Scheme 3.21) [33]. A diverse set of enantioenriched seven‐membered heterocycles attached to spiro‐ε‐lactam oxindoles 225was obtained with excellent enantioselectivities (90–>99% ee), and moderate to good diastereomeric ratios (3.2 : 1 to 13 : 1 dr). The aminoindanol‐derived triazolium pre‐catalyst 226in the presence of DMAP and the oxidant 227revealed the optimal catalytic system for the transformation. Different oxotryptamine derivatives and enals were successfully used as reaction substrates (22 examples) including a β‐alkyl enal, although in the latter case, the corresponding product 235was obtained in lower yield and diastereoselectivity maintaining excellent asymmetric induction.
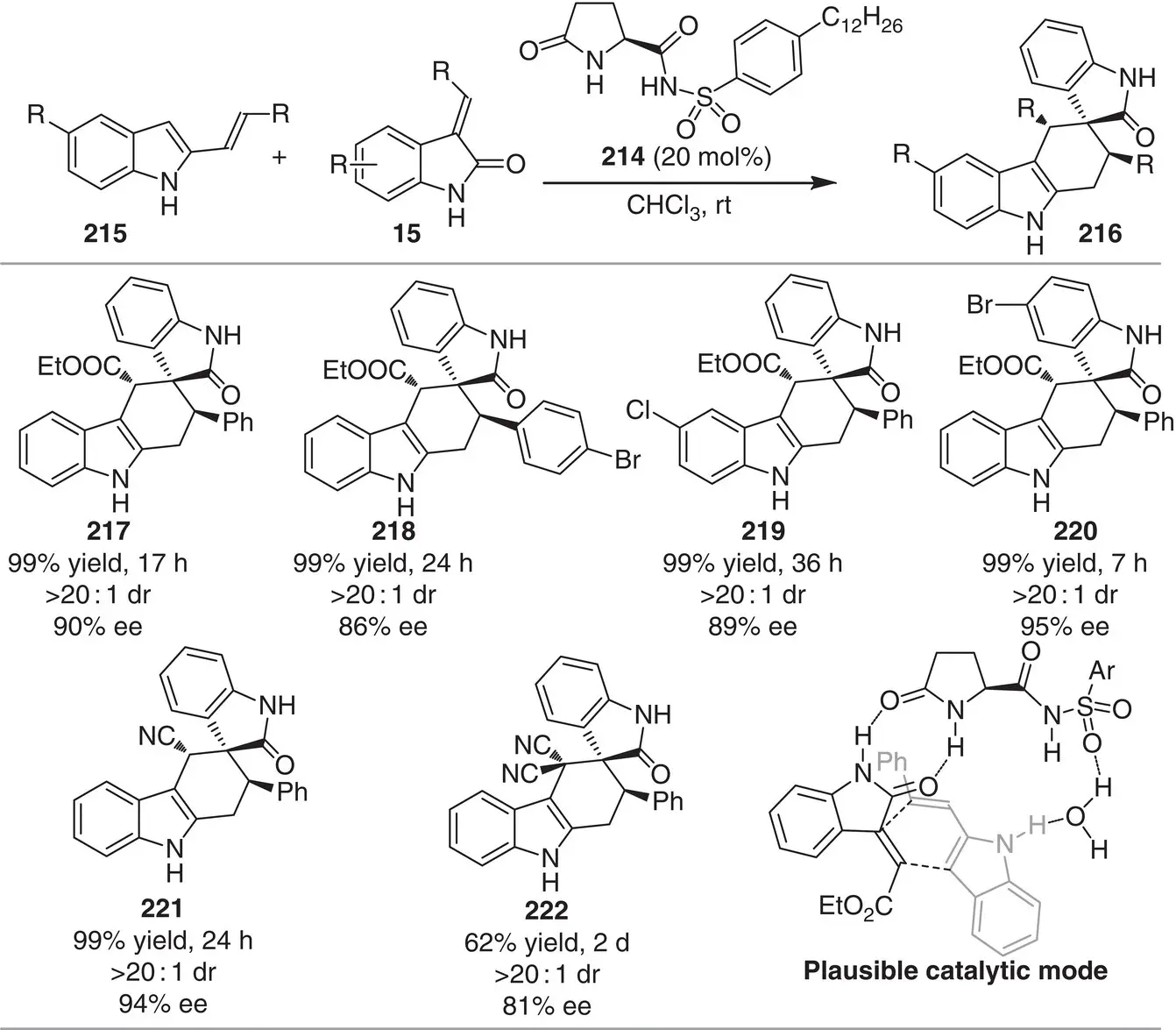
Scheme 3.20 Enantioselective Diels–Alder cyclization for the synthesis of carbazolespirooxindoles.
Source: Modified from Ren et al. [32].
In 2015, Jørgensen and coworkers reported an elegant catalytic methodology for the synthesis of biologically relevant complex spirocyclobutaneoxindole derivatives 236( Scheme 3.22) [34]. The chemistry is based on the organocatalytic enamine‐based activation of cyclopropanes 237, forming a reactive donor–acceptor cyclopropane intermediate that in the presence of 3‐alkylidene oxindoles 238promotes the formal [2+2] cycloaddition reaction to construct the spiranic products 236in good yields, high diastereomeric ratios, and excellent enantiomeric excesses (15 examples, 3.2 : 1–>25 : 1 dr,70–97% ee). In fact, the reaction outcome is surprising since the traditional Lewis acid activation of donor–acceptor cyclopropanes typically promotes the [3+2]‐cycloaddition pathway. After mechanistic studies, the authors propose two possible reaction mechanisms for the spirocyclobutaneoxindole formation. The first plausible mechanism involves the organocatalytic activation of the cyclopropyl functionality leading to the formation of the iminium‐ion intermediate 239by ring opening. From this intermediate, the dienamine 240can be formed by deprotonation. Then, this dienamine intermediate reacts in a formal [2+2]‐cycloaddition with the 3‐olefinic oxindole 238to form the spirocyclobutaneoxindole product 236. An alternative mechanistic possibility considers an initial [3+2]‐cycloaddition between the 3‐alkylidene oxindole and the organocatalytically activated cyclopropane, followed by a ring‐contracting rearrangement. However, the lack of a rational driving force for this rearrangement makes the latter reaction pathway more unlike.
Читать дальшеИнтервал:
Закладка:
Похожие книги на «Spiro Compounds»
Представляем Вашему вниманию похожие книги на «Spiro Compounds» списком для выбора. Мы отобрали схожую по названию и смыслу литературу в надежде предоставить читателям больше вариантов отыскать новые, интересные, ещё непрочитанные произведения.
Обсуждение, отзывы о книге «Spiro Compounds» и просто собственные мнения читателей. Оставьте ваши комментарии, напишите, что Вы думаете о произведении, его смысле или главных героях. Укажите что конкретно понравилось, а что нет, и почему Вы так считаете.