Spiro Compounds
Здесь есть возможность читать онлайн «Spiro Compounds» — ознакомительный отрывок электронной книги совершенно бесплатно, а после прочтения отрывка купить полную версию. В некоторых случаях можно слушать аудио, скачать через торрент в формате fb2 и присутствует краткое содержание. Жанр: unrecognised, на английском языке. Описание произведения, (предисловие) а так же отзывы посетителей доступны на портале библиотеки ЛибКат.
- Название:Spiro Compounds
- Автор:
- Жанр:
- Год:неизвестен
- ISBN:нет данных
- Рейтинг книги:3 / 5. Голосов: 1
-
Избранное:Добавить в избранное
- Отзывы:
-
Ваша оценка:
- 60
- 1
- 2
- 3
- 4
- 5
Spiro Compounds: краткое содержание, описание и аннотация
Предлагаем к чтению аннотацию, описание, краткое содержание или предисловие (зависит от того, что написал сам автор книги «Spiro Compounds»). Если вы не нашли необходимую информацию о книге — напишите в комментариях, мы постараемся отыскать её.
A comprehensive treatment of the latest research in, and applications of, spiro compounds Spiro Compounds: Synthesis and Applications
Spiro Compounds
Spiro Compounds — читать онлайн ознакомительный отрывок
Ниже представлен текст книги, разбитый по страницам. Система сохранения места последней прочитанной страницы, позволяет с удобством читать онлайн бесплатно книгу «Spiro Compounds», без необходимости каждый раз заново искать на чём Вы остановились. Поставьте закладку, и сможете в любой момент перейти на страницу, на которой закончили чтение.
Интервал:
Закладка:
In the reported reaction mechanism, a rhodium(I)/( S )‐binap cationic complex is responsible for the excellent stereocontrol (96%–>99% ee), affording the spiro cyclohexadiene products 123in moderate to good yield (31–76%). Mechanistically, the reaction proceeds through the formation of intermediate 127by coupling the two terminal alkynes 124and 125with cyclopropylideneacetamides 126in the presence of Rh(I) complex. Regioselective insertion of 126produces the intermediate 127which undergoes direct reductive elimination to furnish the final spiranic product 123. In a subsequent report, the authors extended the scope of the [2+2+2] cycloaddition to 1,6‐enynes instead of alkynes [24].
In 2018, the group of Rios reported an enantioselective synthesis of spiropyrazolones 135by a formal [6+2] cycloaddition reaction ( Scheme 3.13) [25]. The method relies on the catalytic synergistic activation of the seven‐membered pyrazolone derivatives 136by Pd along with the activation of enals 137by the chiral secondary amine 138. Specifically, the reaction is initiated by the simultaneous formation of highly active Pd‐complex 139and the chiral iminium ion intermediate 140. Stereoselective interception of the iminium ion by the nucleophilic Pd‐complex 139provides the enamine intermediate 141bearing the Pd‐coordinated allyl cation. Intramolecular ring closing between the enamine moiety and allyl cation in 141furnishes the final spiranic products 135. The [5,5]spiropyrazolone products are obtained in good yields (54–79%), moderate to good diastereoselectivities (3.2 : 1 to 20 : 1), and excellent enantiocontrol (93%–>99%).
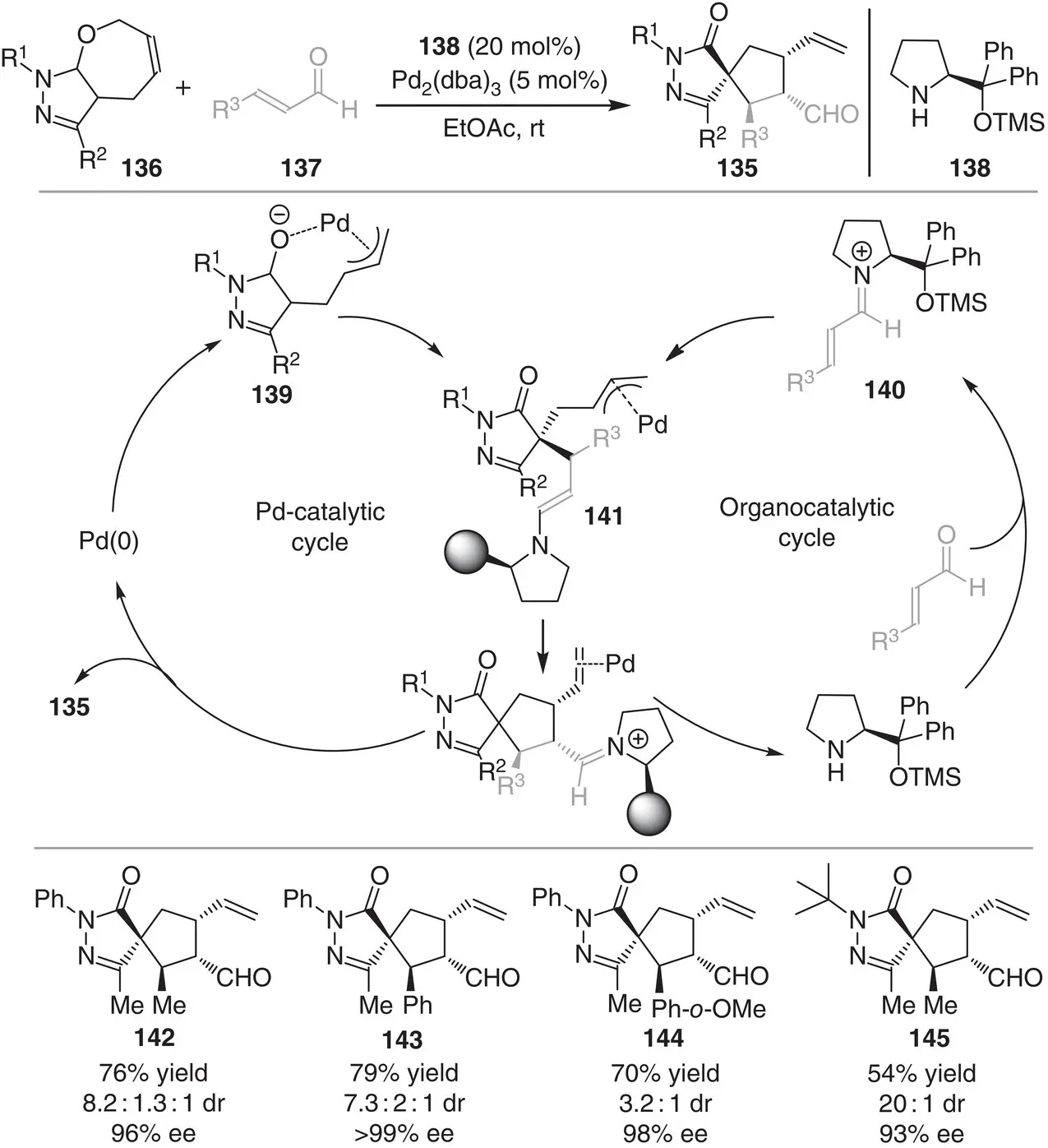
Scheme 3.13 Synergistic palladium/chiral secondary amine‐catalyzed formal ring contraction for the enantioselective synthesis of spiropyrazolones.
Source: Modified from Meazza et al. [25].
3.3 Organocatalytic Methodologies
The versatility and robustness of the different organocatalytic activation modes have been implemented in a number of stereoselective cascade processes, addressing the requests of atom and step economy of modern synthetic chemistry. Remarkably, organocatalysts are generally robust, readily available, and their easy scaffold modifications allow the straightforward generation of different structural variants for the development of highly diversified asymmetric transformations. In this scenario, organocatalytic methodologies represent a powerful strategy for the preparation of complex spiro compounds with high optical purity. The section has been divided into four subsections according to the involved reactions: [3+2] cycloadditions, [4+2] cycloadditions, [4+3]‐, [2+2] cycloadditions and switchable strategies, and miscellaneous reactions.
3.3.1 Organocatalytic [3+2] Cycloaddition Strategies to Construct Spiro Compounds
The group of Glorius envisaged an a 3–d 3umpolung reactivity strategy of β,β‐disubstituted enals to construct spirocyclic oxindoles 146bearing two highly congested contiguous quaternary carbon centers ( Scheme 3.14) [26]. In this manner, the diastereoselective annulations of a variety of isatins 147with β,β‐disubstituted enals 148were successfully developed under N ‐heterocyclic carbene catalysis 149. The use of a pivalic acid as Brønsted acid cocatalyst revealed crucial in order to ensure high yields and diastereoselectivities (8 : 1–>20 : 1 dr and 68–98% yields). Moreover, the authors carried out an enantioselective variant using a chiral NHC catalyst where the acid cocatalyst also had a beneficial effect on the reaction enantioselectivity (one example, 84% ee). The authors proposed that after the formation of Breslow intermediate, the acid cocatalyst stabilizes the intermediate 150 via two hydrogen bonds, promoting the diastereoselective formation of the adduct 151. Subsequently, intramolecular attack of the alkoxide moiety at the carbonyl group in 151affords the desired spirooxindole 146and regenerates the NHC catalyst.

Scheme 3.14 Conjugate umpolung of β,β‐disubstituted enals and isatins promoted by N ‐heterocyclic carbene/Brønsted acid dual catalysis.
Source: Modified from Li et al. [26].
Substrates that bear multiple nucleophilic and electrophilic sites are highly useful syntons to rapidly generate architecturally complex molecules and bioactive compound libraries. In this context, Sun and coworkers reported a method for the construction of five‐membered spirocyclic oxindoles 160based on a Michael–Mannich cascade reaction of a ketimine intermediates, characterized by a dichotomous reactivity profile, with methyleneindolinones 1( Scheme 3.15) [27]. The one‐pot process is catalyzed by the bifunctional quinine‐derived squaramide 161in an enatioselective fashion. Several β‐dicarbonyl substrates 162bearing different alkyl chains and both electron‐withdrawing and electron‐donating groups on the aryl ring of the methyleneindolinone 1and the nitrosoarene 163are well tolerated (31–94% yields, >20 : 1 dr and 95–99% ee). Also, various electron‐withdrawing groups (R 4) at the methylene position afforded the desired products with high stereocontrol. Control experiments pointed out the importance of the Boc group as a hydrogen‐bond acceptor for activating the methyleneindolinones. The proposed mechanism is depicted in Scheme 3.15; enolization of acetylacetone promoted by the tertiary amine group of the bifunctional catalyst is followed by N ‐selective addition to nitrosobenzene under basic conditions. The resulting ketimine is deprotonated and the methyleneindolinone is activated by the same catalyst to promote the intermolecular Michael addition. The final irreversible Mannich‐type cyclization proceeds smoothly to afford the enantioenriched spiranic products 160.
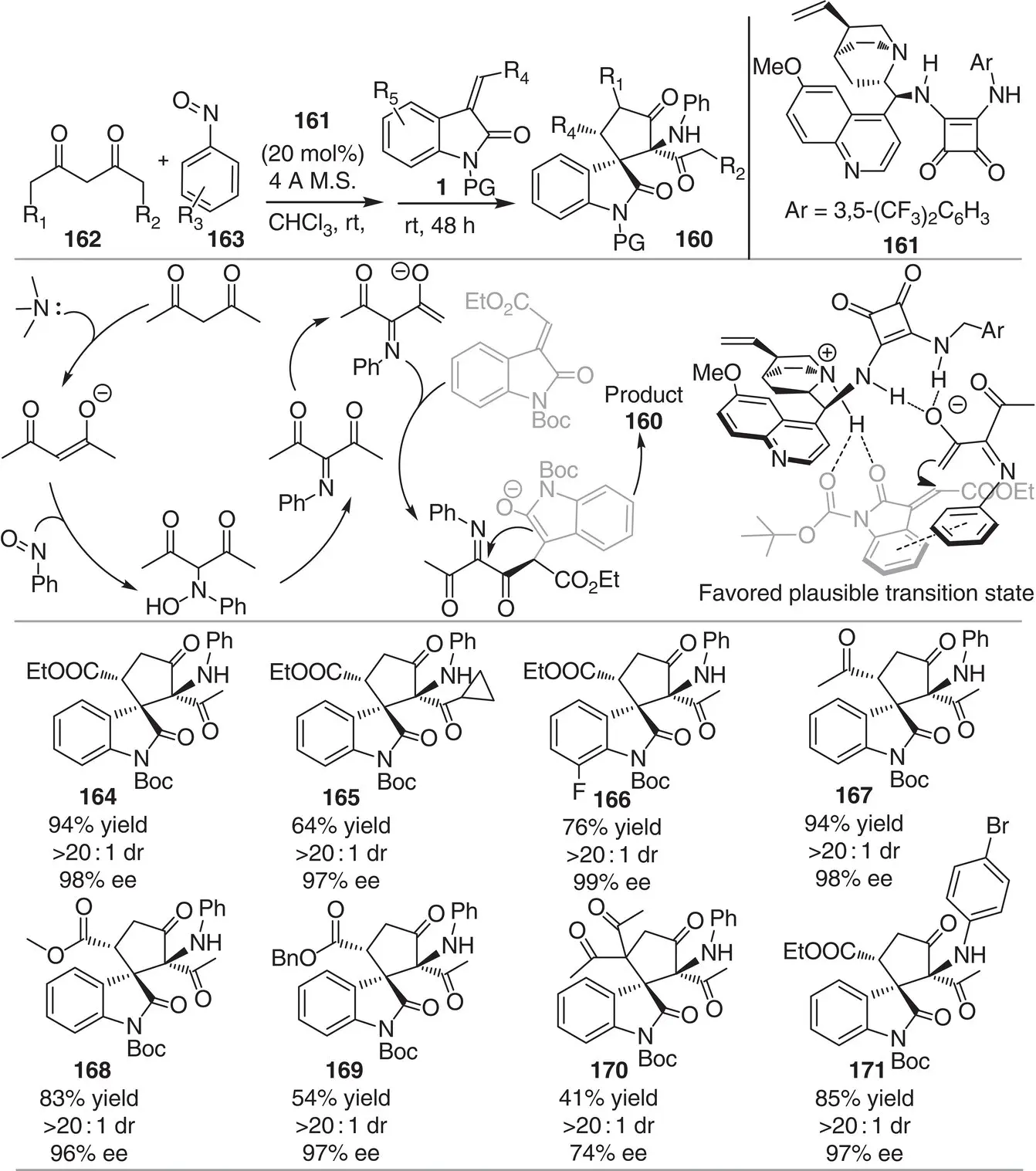
Scheme 3.15 Bifunctional squaramide‐catalyzed synthesis of enantioenriched spirocyclic oxindoles via ketimine intermediates with multiple active sites.
Source: Modified from Sun et al. [27].
Wang, Hong, and coworkers reported the first stereoselective 1,3‐dipolar cycloaddition reaction between azlactones 172and methyleneindolinones 1catalyzed by chiral phosphoric acids ( Scheme 3.16) [28]. The methodology gives access to highly substituted 3,3‐pyrrolidonyl spirooxindole scaffolds 173, which are present in a number of natural products. The authors take advantage of the high versatility of azlactones, which under the effect of Brønsted acids can form N ‐protonated 1,3‐dipoles. The dipole reacts with the methyleneindolinone 1wherein the hydroxyl proton and the phosphoryl oxygen of the chiral phosphoric acid form double H‐bond interactions with both substrates, channeling the reaction in an enatioselective manner. The substituents on the binol backbone of the catalysts have a significant influence on the reaction enantiocontrol, where the bulkier catalyst 174gave the best results. A wide variety of substitution patterns and functional groups on both substrates are well tolerated, affording the products 173with good to excellent yields, diastereo‐ and enantioselectivities.
Читать дальшеИнтервал:
Закладка:
Похожие книги на «Spiro Compounds»
Представляем Вашему вниманию похожие книги на «Spiro Compounds» списком для выбора. Мы отобрали схожую по названию и смыслу литературу в надежде предоставить читателям больше вариантов отыскать новые, интересные, ещё непрочитанные произведения.
Обсуждение, отзывы о книге «Spiro Compounds» и просто собственные мнения читателей. Оставьте ваши комментарии, напишите, что Вы думаете о произведении, его смысле или главных героях. Укажите что конкретно понравилось, а что нет, и почему Вы так считаете.