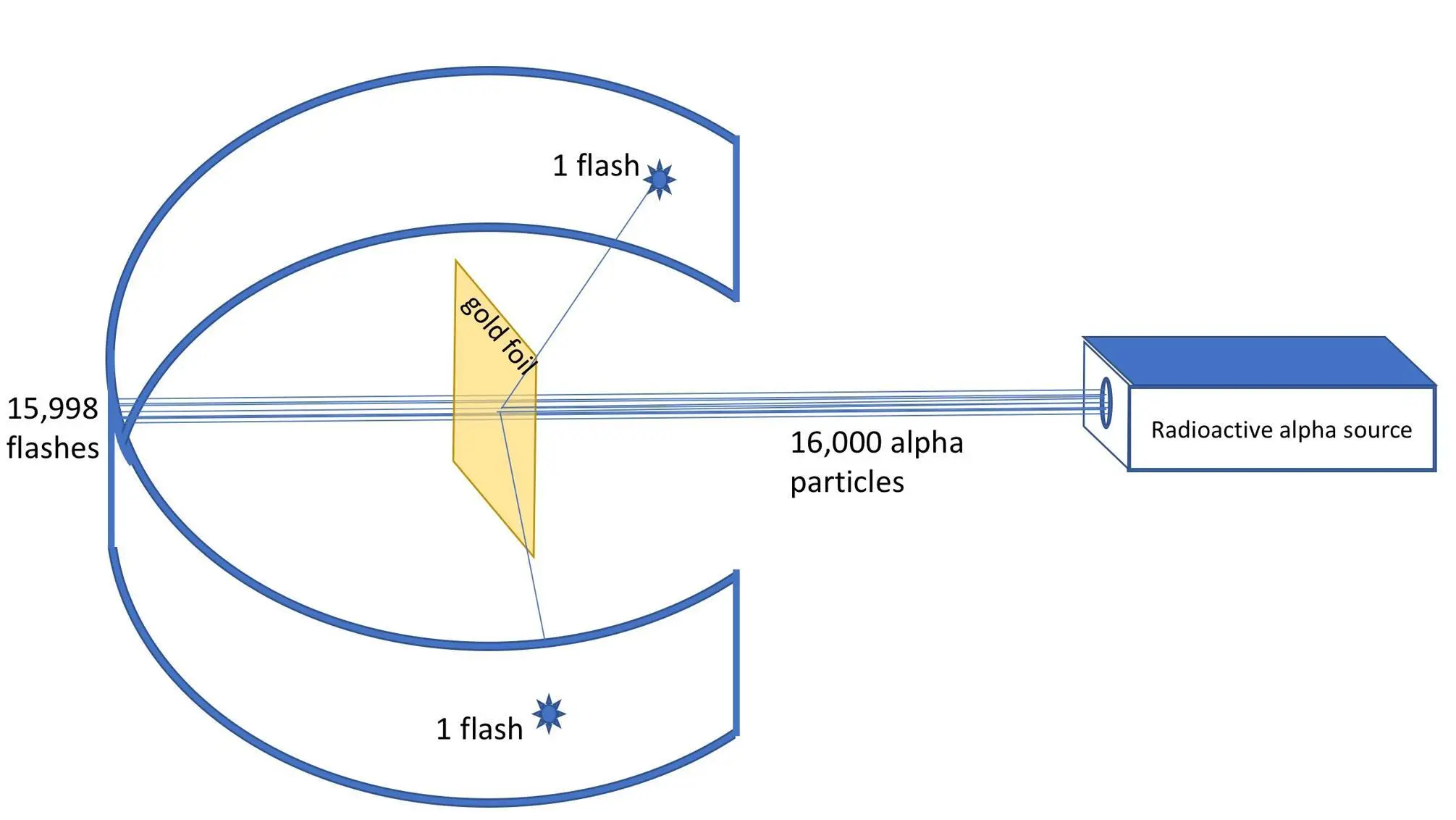
Figure 4.5: Shooting alpha particles from a radioactive source through an ultrathin gold foil. Only 1:8,000 particles will be deflected.
A gold atom has a diameter of 0.3 nm, so such a 210 nm thick gold foil will still be around 800 atoms thick when we assume closely packed spheres. From the perspective of these utter tiny alpha particles, the gold foil should therefore be experienced as an impressive solid 800 gold atoms thick wall.
According to Thomson’s "plum pudding” atom model such a solid wall should present a major obstacle for the massive alpha particles. However, the experimental result turned out quite contrary to expectations. Almost all alpha particles shot straight through the foil as if that solid wall of 800 heavy gold atoms thick did not exist at all. But some were deflected. Of 8,000 alpha particles, an average of 1 was deflected and sometimes even completely bounced straight back. The hits were measured by counting the little light flashes made by the alpha particles when they hit a zinc-sulfide screen, that could be moved on a circular rail. Rutherford assumed that those rare deflections were in fact near-collisions of positive alpha particles with incredibly small positive atomic nuclei. He is said to have exclaimed:
“It was almost as incredible as if you fired a 15-inch shell at a piece of tissue paper and it came back and hit you.”
Rutherford concluded that the atom had to consist of negatively charged electrons orbiting a very small and positive nucleus. The inside of an atom was virtually empty space. He was able to calculate from the ratio of the deflected alpha particles against the not deflected ones – 1:7999 – that the ratio of the small nucleus to the size of the electron shell should measure approximately between 1:10,000 to 1:20,000. An image now emerged of atoms as miniature solar systems with minuscule negatively charged electrons, orbiting like tiny satellites the positively charged nuclei at high speeds. To give you an impression of the proportions of the Rutherford atom: the ratio of the nucleus to the electron shell can be compared with the ratio of a fruit fly of 2 to 4 mm to the dome of St. Peter's in Rome. Take a moment to visualize that image of a fruit fly hovering in the middle of that dome. So, 99.999999999% of the atom is just empty space, void. The solidity of matter started more and more to look like an illusion. But even still stranger views were soon to come.
The reason why we do not sink through a floor consisting of these ephemeral atoms, which we now know as mainly empty space, has to do with the Pauli exclusion principle [1 6 ], discovered by Wolfgang Pauli(1900-1958). This principle prohibits electrons from having the same position in space when all their quantum properties have equal values. These properties are connected to the four quantum numbers that describe the quantum state of the electron. One of them is the electron spin. The electron spin is the quantum version of the north-south orientation of a magnet. It was discovered in 1925 by two Dutch PhD students – George Uhlenbeck(1900-1988) and Samuel Goudsmit(1902-1978). They were awarded the Max Planck medal in 1964 for their discovery of the electron spin. The electrons in the outer shells of the atoms of our hand repel the electrons from the outer shells of the atoms of the wall, and vice versa. Their absolute refusal to have the same position in space when their quantum numbers are the same, prevents us putting our hand through a wall, where both hand and wall are in fact almost entirely made up of empty space. The electrons of these atoms repel each other, the nearer to each other the stronger. So, in fact, we don't even touch the wall, even when we hit it hard.
From Rutherford comes the parable of the two desks. One desk is solid and exists in the solid world of our daily experience, the other desk is mostly empty space, ghostly and exists in the world of physical ideas. Which desk would be the real one? What is your opinion?
The solar system model of the atom is impossible
“We live on an island surrounded by a sea of ignorance. As our island of knowledge grows, so does the shore of our ignorance.”
John Archibald Wheeler, 1911 – 2008
The classical idea of electrons as little charged particles orbiting a positive core encountered serious problems:
Why are the orbits of the electrons around the atom not circular like planetary orbits, but spherical? The hydrogen atom with only a single electron in a circular orbit should be flatter than a dime. But spherical orbiting electrons which would continually have to change their courses, buzzing around like annoying flies around your head, are even more inconceivable.
Is it possible that the circular orbit of the electron slowly changes its tilt thus creating a sphere of ephemeral presence? This would be unstable with heavier atoms having multiple electrons repelling each other.
The image of electrons buzzing around, all of them together in the outer shell of any atom heavier than hydrogen, represents a highly unstable atom shell since they all will repel each other.
Why is it that orbiting electrons, where orbiting means that they are continually changing their velocities, do not lose their kinetic energy, as Maxwell equations predict, through the emission of electromagnetic radiation to eventually spiral down into the core?
The last-mentioned problem follows immediately from Maxwell's classical electromagnetic theory. A circularly moving charge is technically an alternating electric current. Alternating currents generate changing electric fields and therefore EM-waves. That is the principle of every radio transmitter. Emitting EM-waves means loss of energy. The moving electron should therefore quickly lose its kinetic energy in an extremely short time.
In fact, the number of questions kept increasing instead of decreasing after each new discovery. Incidentally, this expanding sea of unknowns is nowadays still the case, and it is not expected that this will change in the foreseeable future.
Energy is equal to mass according to Einstein
In setting up his special theory of relativity in 1905, Einstein derived the equivalence of mass and energy: E = mc 2 [1 7 ]. The meaning of this simple but astounding formula is often interpreted as the idea that mass can be converted into energy (and vice versa). But Einstein's own interpretation is that mass and energy are two sides of the same coin. That means literally that an amount of energy has also a corresponding amount of mass. A small lump of matter therefore represents an enormous amount of ‘solidified’ energy. A mass of 1 kg is equal to an energy of 9x10 16J. Just compare this with the total annual energy consumption of The Netherlands: 3.1 x 10 18J: an energy equivalent to about 35 kg of mass. The theory says also that the mass of an accelerated object increases with its increasing speed. Some form of energy must be transferred to the object to accelerate it. The kinetic energy, the energy that a moving object possesses by its movement, will increase and therefore, conform E = mc2, also its mass.
The increasing mass with increasing velocity is an effect that must be seriously taken into account in large particle accelerators, such as the CERN Large Hadron Collider in Geneva. Protons are accelerated to enormous speeds, very close to the speed of light – and therefore also to considerably larger masses. Because of this increase in mass, they need a correspondingly adjusted strong magnetic force field to keep them in their precise circular orbits. At particle speeds very close to that of light, the supercooled magnets used to hold the particles in their orbits must be continually and very precisely adjusted to exert the required larger centripetal forces. But in our everyday environment this effect exists too. E = mc 2means that a fully charged AA NiMh cell will have an extremely minute amount of mass increase compared with the discharged state. Something in the order of 10 - 10grams.
Читать дальше













