Now for the problem that Einstein solved. The photoelectric effect is the phenomenon that, under the condition that the light frequencies are above certain values, light shining on a metal surface will release electrons from the metal. This limit was in obvious contradiction with Maxwell’s continuous EM-waves. The frequency of the light was the decisive prerequisite for the occurrence of the effect, not the intensity. So, for example red light – low frequency – did not release electrons, no matter how high the intensity of the red light was. Like when you cannot fill a glass from a tap with a wide opening, from which the water flows broadly with little force, but is filled easily from a narrow spout from which the water shoots with great force. This begged for an explanation, but classical Newtonian physics – more specifically Maxwell's equations – did not provide an explanation. That is because according to classical theory any EM wave of any frequency could provide enough energy when applied long enough.
Einstein's solution [ 8 ]of the problem was that light had to be quantized in particles, later called photons, according to Planck's formula: E =h.f. The energy for each light particle had to be above a certain minimum value to be able to release an electron from the metal. Releasing an electron from metal needs a minimum amount of energy. Einstein’s paper was therefore a fair confirmation of Planck's quantum hypothesis. A single photon with the right frequency – and therefore sufficient energy – could release one electron, but a whole bunch of photons of a low frequency achieved nothing, although their combined energy would be more than sufficient. You could regard these light particles as a rehabilitation for Newton's corpuscles, where each particle has its specific color – which is now also its frequency. Photons are therefore considered as discrete energy packages.
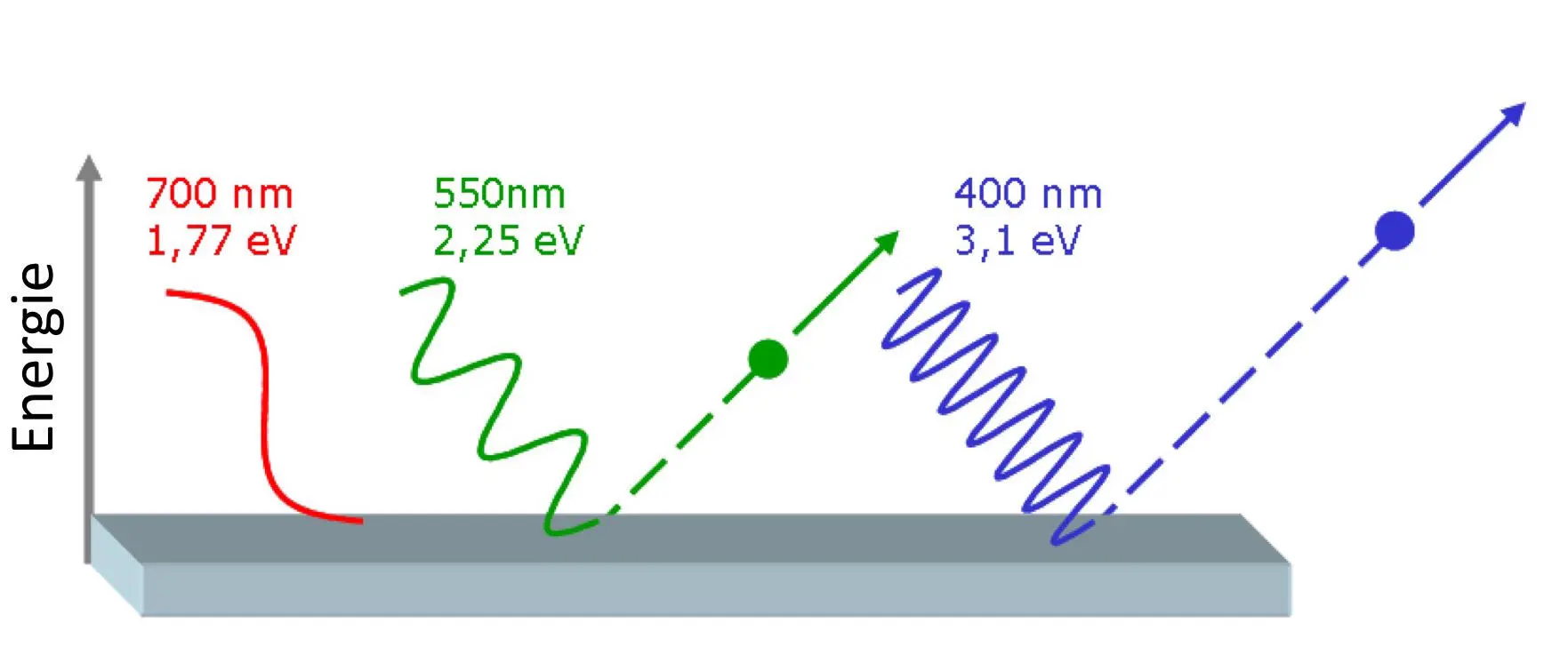
Figure 4.3: Photoelectric effect. Red light photons have too little energy, 1.77 eV, to release an electron from the metal. Green light has just enough energy, 2.25 eV. Violet light has more than enough so that the electron shoots away with a kinetic energy of 3.1-2.25 eV = 0.85 eV. Source: Wikipedia.org
The physical significance of the frequency and wavelength of a photon was not clear, and this has not become clear today yet. We cannot imagine properly a particle that is at the same time a wave of a single frequency. It has been tried. Just try to visualize water waves on a lake that are concentrated in little wavy blobs, each with its own frequency and energy. An impossible task for our imagination.
How a spherical expanding electromagnetic Maxwell wave, whereby the energy also spreads out over the same spherical shape, becomes the very localized and precise energy transfer of the electromagnetic quantum, could not be explained by any means. The surface of the expanding sphere will increase with the square of the distance to the source. The energy per square inch of the wave will therefore decrease in an inverse square ratio to the distance from the source. To test this, just walk towards a bright light with a camera with its exposure setting in the A-mode ( fixed aperture [ 9 ]) and observe the inverse quadratic relationship between distance and shutter speed.
According to the very local discrete energy transfer, that is the photon, when it releases an electron in the photoelectric effect, this spherical distributed EM-wave would have to alter suddenly into a single discrete photon. This energy transfer per photon only depends on its frequency and not at all on its distance from the source. This is clearly in conflict with a spherical continuous expanding energy distribution. Maxwell's EM wave and Einstein's photon are both very useful models that, however, clearly cannot be reconciled with each other, at least not on the scale of atomic interactions. This irreconcilability is a profound and still unsolved enigma since Planck's discovery of the quantum of energy transfer and Einstein's introduction of the light particle. This mystery has still not been solved to satisfaction until today.
Although Einstein with this publication made an enormously important contribution to quantum physics, he has since then, during almost his entire career, fiercely protested against the implications of quantum physics, mainly because these clearly contradicted causality, a basic assumption in classical physics, and also strongly suggested that his relativity laws were violated. His relativity laws told that the speed of light was the maximum speed of information exchange in the universe. The theory of relativity is convincingly confirmed by numerous experiments. Were relativity not implemented in our GPS systems, their indications would deviate in an important way from our actual locations.
Planck limited his concept of quantization to transmitted and absorbed energy, but Einstein proposed that every expanding EM-wave was quantized in particles, photons. That subtle difference may seem unimportant, but it isn’t. More about that later, as we will discover that photons are probably just reified abstract mathematical concepts.
In summary, electromagnetic radiation was found to have evidential wave-like properties, but also evidential particle properties.
Meanwhile, the construction of matter on its smallest scale was investigated in various laboratories. Ernest Rutherford did a ‘golden’ discovery.
Ernest Rutherford’s model of the atom
While doing experiments with cathode rays in vacuum glass tubes – the precursors of the once ubiquitous but nowadays outdated TV display tube – and directing these rays through an electric or a magnetic field, John Joseph Thomson(1856-1940) noticed that these rays were deflected by those fields. See figure 4.4.
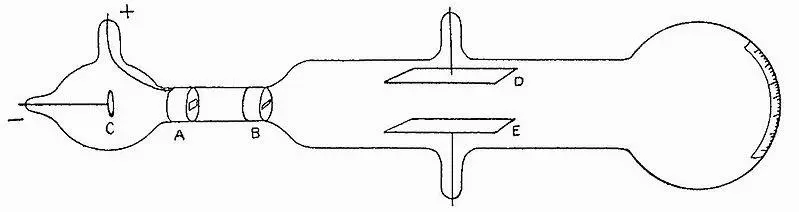
Figure 4.4: Vacuum tube for cathode ray deflection by an electric field measurement. Plates D and E are electrically opposite charged.
Source: J.J. Thomson – Philosophical Magazine 1897.
Thomson inferred from their deflection in electric fields that cathode rays had to be composed of negatively charged particles. He dubbed these particles electrons. He assumed then that these electrons existed in matter and were evenly distributed within the atoms that made up matter. This made an atom into a positively charged solid little ball with negative electrons scattered through it, like currants in a plum pudding. Concerning his “plum pudding” model Thomson apparently did not realize that, because of their mutual repulsion, all electrons should be evenly distributed on the outside of the atom, each as far away as possible from the others. Also, the magnitude of the electric charge of the electron was still unknown at time of Thomson’s discovery. The electron charge was later determined by Millikan [1 0 ]by measuring with a microscope the velocities of slowly falling electrically charged oil droplets, a beautiful, sophisticated laboratory experiment.
Experiments [1 1 ]conducted under supervision of Ernest Rutherford [1 2 ](1871-1937) revealed that electrons probably formed the outer ‘hard’ shell of the atom with an almost totally empty void inside. In the centre of that emptiness resided an utterly tiny positive mass – the nucleus. For an electrically neutral atom the positive charge of this nucleus had to be equal to the sum of the negative electron charges. In 1908 Rutherford’s assistants Marsden and Geiger [1 3 ]shot alpha rays – positively charged and fast moving alpha particles, ejected at high velocity from a radioactive source (radium) – at a gold foil. As Rutherford already suspected, these alpha particles turned out to be helium nuclei, consisting of two protons and two neutrons. Gold can be beaten to an ultrathin foil [1 4 ]of less than 1 nm thickness (1 nanometer = 0.000000001 m) but Marsden and Geiger used a gold foil of around 210 nm thick [1 5 ]in their experiment.
Читать дальше














