Recent Developments Associated with the Microrough Surfaces
Attempts to improve the wettability of the implant surface
In recent years, attempts have been made to improve the wettability of the implant surfaces. The surface wettability of dental implants appears to have a significant impact on the biologic cascade of events that occur at the bone-implant interface and is modulated by the chemistry and topography of the implant surface. 65It has been shown that the wettability of the implant surfaces plays an important role in the adsorption of plasma proteins 66and the differentiation and cell adhesion of mesenchymal stem cells into bone-producing cells. 67
Several methods have been used in an attempt to increase the wettability of the implant surface, including the application of fluoride ions and magnesium ions to the implant surface. This is referred to as electrowetting and allows the plasma proteins to flow freely onto the implant surface and into the irregularities of the microroughened surface immediately upon insertion of the implant. Moreover, fluoride ions on the implant surface, when applied to a titanium grit-blasted surface, increase the expression of the genes associated with the differentiation of osteoprogenitor cells 68– 70and promote osseointegration during the early stages of healing. 71
Another approach is to package the freshly prepared titanium implants in saline. Packaging the implants in this manner reduces the rate of contamination of the surface of the implant, which maintains the surface energy and wettability. Recent studies have also shown that photofunctionalization will increase the wettability of the implant surface.
Genetically engineered implant surfaces
Since the discovery of osseointegration-specific genes, it has been an inviting idea to imbed one or more of these genes onto the surface of the implant. There are several advantages to this approach. The genes do not degrade in these environments and can be applied to the implant surfaces in suitably low doses. Moreover, these genes are associated with the normal cell cascade of cell differentiation and function. However, there are significant disadvantages. The primary disadvantages are the cost of development and the regulatory issues that must be addressed before bringing such a product to the marketplace. Unless there is a significant clinical advantage to be gained using gene-enhanced implant systems, the cost of ensuring safety and efficacy outweighs the benefits.
Nanoenhanced, biomimetic implant surfaces
The surface of bone is highly mineralized with HA crystals and exhibits a highly complex surface topography. During the process of bone remodeling and following acid etching by osteoclasts, bone possesses distinct surface chemistry and topography. Since the phenomenon of osseointegration was introduced in the late 1970s, researchers have attempted to create so-called “biomimetic” implant surfaces; in essence, implant surfaces with surface chemistry and topography that mimic that of acid-etched bone surfaces found in vivo. Furthermore, submicron-to-nanometer titanium surface features enhance cytocompatibility properties for bone-forming cells, increasing both surface energy and cell adhesion. 72
The two methods developed during the last several years—crystalline deposition of HA crystals on microrough surfaces of titanium implants 18and the application of pico-to-nanometer-thin TiO 2coating on microroughened titanium surfaces 73—are the most recent attempts to mimic the physical and chemical environments found in vivo (see later in the chapter). These nanoenhanced surfaces optimize platelet activation, facilitate the adsorption of plasma proteins, and accelerate differentiation of mesenchymal stem cells into functioning osteoblasts. Furthermore, gene expression of the differentiating osteoprogenitor cells is accelerated and upregulated. 18, 22, 74The result is that the events associated with osseointegration are substantially accelerated.
Implant surfaces enhanced with recombinant peptides
Application of recombinant osteogenic proteins (OP-1), bone morphogenetic proteins (BMP2, BMP7), and growth factors such as platelet-derived growth factor (PDGF) to the surfaces of implants has been of interest because of the potential of enhancing osteoconductive properties of the implant surface. However, the optimal means of bonding these proteins to implant surfaces have not been determined. In addition, retention and controlled release of these proteins has been difficult. Additional disadvantages are the fact that many of these proteins are costly, they frequently are not associated with the normal cellular cascade, and they may be deactivated during sterilization of the implant. In addition, higher concentrations/doses of BMP2 have triggered troublesome side effects. 75
It is possible to bind these proteins to implant surfaces. Implants with HA and chitosan coatings have been used most often. The outcomes of most of these studies indicate that binding BMPs to the implant surface significantly enhances osseointegration. 76, 77Other researchers 75, 78have attempted to determine whether coatings of osteogenic proteins can be used effectively for vertical augmentation of deficient ridges. In an animal study, WikesjÖ et al 75showed that coating porous titanium implant surfaces with rhBMP 2 induced significant bone formation around the neck of the implants, leading to a clinically significant vertical augmentation of the alveolar ridge. In another study using an animal model, Susin et al 78achieved a similar result with rhBMP 7.
Crystalline deposition of HA crystals
Techniques have been reported whereby nano-sized crystals of HA of a specific size (20 Nm) are deposited onto the surface of titanium implants that have previously been double acid-etched (DAE). 18A highly specific distribution and spacing of these crystals can be achieved ( Fig 2-10). 79These crystals are joined to the TiO 2surfaces with covalent bonds. Based on microCT, the amount of BIC associated with nanoenhanced DEA surfaces is equivalent to the BIC of bone deposited onto untreated DEA surfaces. However, the shear bonding strengths needed to separate the implant from the bone anchoring the implant is increased dramatically. The shear bonding levels were more than doubled as compared to DEA surfaces and increased by more than 7 times compared to machined surfaces. Although the effect of crystal deposition was apparent when used on machined-surface implants, the effects were considerably more significant when used in combination with DEA microroughened surfaces, indicating a potential synergistic effect between the two surface phenomena 18, 79( Fig 2-11).
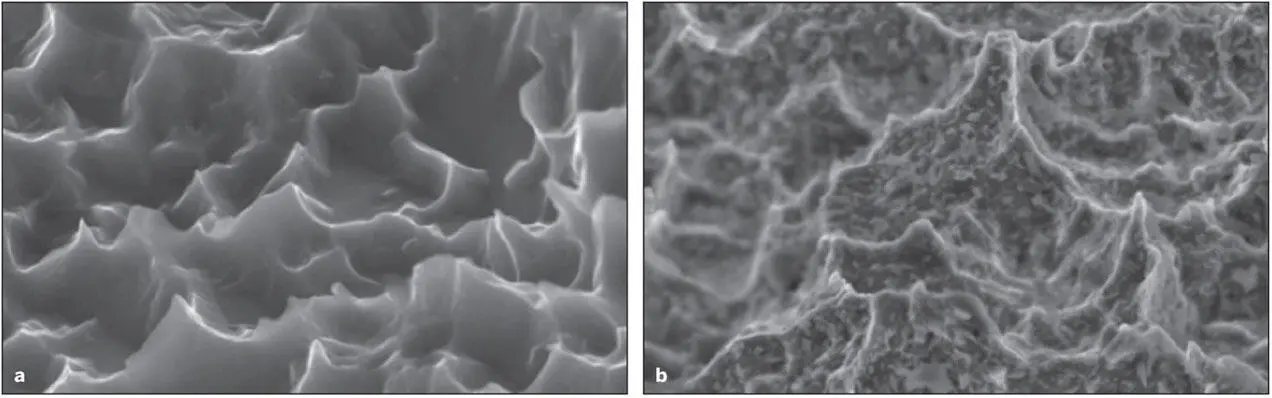
Fig 2-10 (a) A DAE titanium implant surface. (b) A DAE titanium implant surface following depositions of nano-sized HA crystals. (Reprinted from Moy et al 79with permission.)
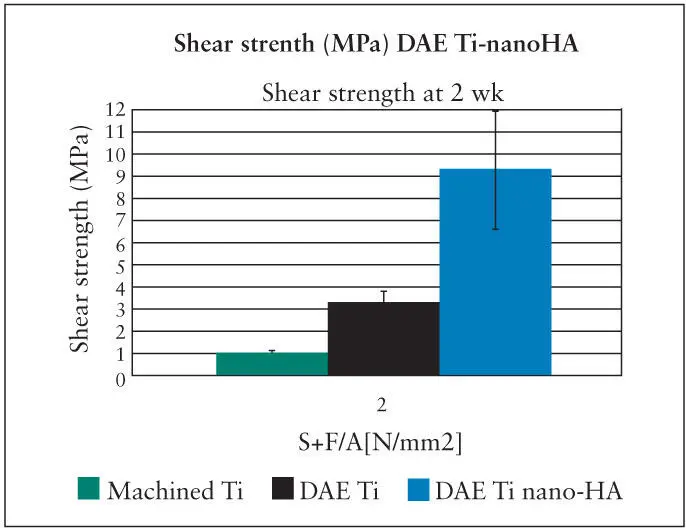
Fig 2-11 The shear strength of the bone-implant interface is dramatically increased following deposition of HA crystals on the surface of a DAE titanium implant. (Reprinted from Moy et al 79with permission.)
As mentioned previously, the hardness of peri-implant bone deposited on microrough implant surfaces is increased progressively from 2 weeks to 4 weeks after surgical implant placement and eventually approaches the hardness equivalent of cortical bone. Of interest was the fact that the hardness of peri-implant bone almost doubled when the moderately rough (sandblasted/acid-etched) implant surface was further modified with nano-HA coating. 30The reasons for this finding have yet to be clearly elucidated.
Читать дальше