With the use of genetic characterization methods, the increased expression of P4H and CRTAP has been reported in the peri-implant tissue during the early stages of osseo- integration. 1, 36While type I collagen gene expression is not significantly affected by the presence of implant materials, the increased presence of collagen cross-linking enzymes associated with the implant is thought to contribute to the formation of stronger peri-implant bone 29, 37( Fig 2-6).
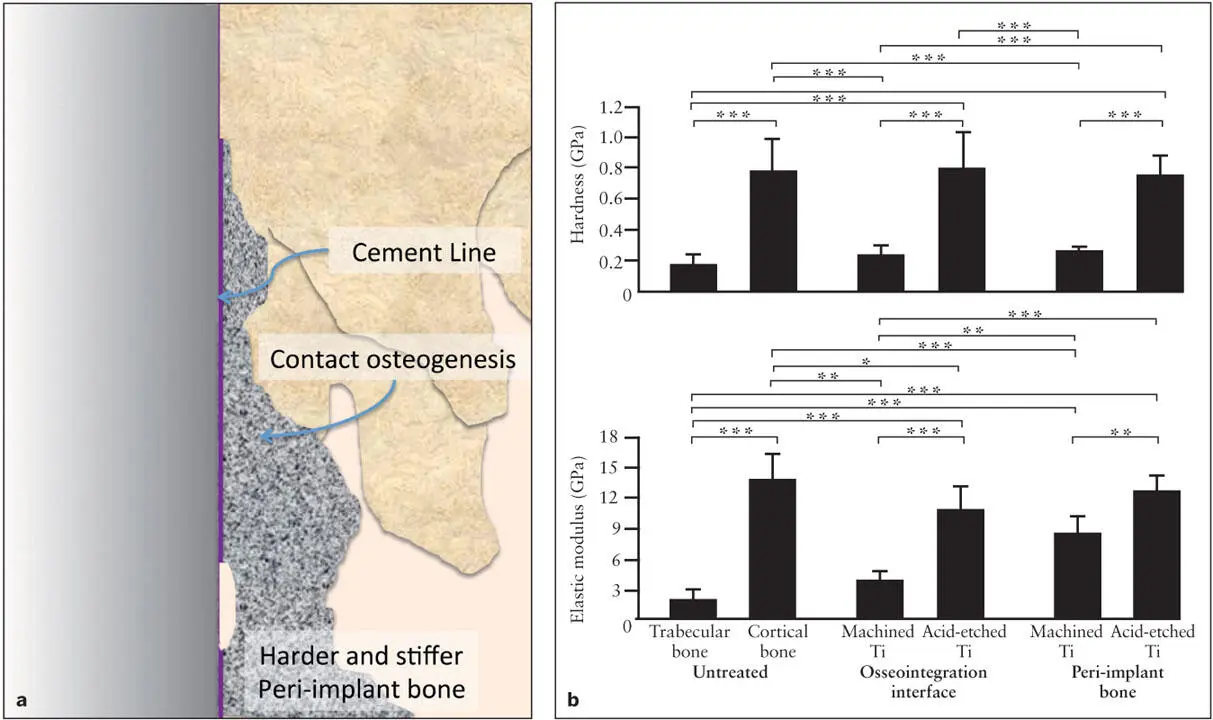
Fig 2-6 (a) Responding to a titanium implant, peri-implant bone synthesized through contact osteogenesis acquires a unique biomechanical property. (b) Hardness and stiffness of bone formed around an implant with a machined or a double acid-etched surface were measured by a nanoindentation assay. Peri-implant bone of the roughened implant was much harder and stiffer than trabecular bone, and its biomechanical properties nearly resembled that of cortical bone. Peri-implant bone deposited on the smooth, machined implant was not as hard; however, it had increased stiffness. * P < .05; ** P < .01; *** P < .001. (Reprinted from Butz et al 29with permission.)
Bone-to-implant contact and interfacial shear strength
Direct bone attachment to the implant surface is the hallmark of osseointegration. Therefore, histologic assessment of osseo- integration commonly uses the percent area of BIC. Higher failure rates in the posterior maxilla have been attributed to its relatively poor trabecular structure leading to decreased BIC. Traditionally, nondecalcified histologic ground specimens have been used to determine BIC. Significant intrasample variations in BIC have been found, 38and a small but critical discrepancy has also been reported between histologic specimens and 3D images reconstructed through microcomputed tomography (microCT). 39Therefore, the data analysis of BIC may require careful interpretation.
Recently, an increasing number of studies report that BIC does not correlate with mechanical failure. When the implant push-in test and microCT-based 3D BIC were used in a rat model, the moderately rough implant (due to double acid etching) showed three times higher shear strength than the relatively smooth machined implant. 40Because the 3D BIC was not different between these tested implants, the increased interfacial shear strength was due to the increased bone bonding to the implant surface. The mechanical interlocking mechanism for roughened implants may contribute to the increased withstanding load. However, this study indicated that epoxy resin–embedded implants showed only a small increase in the withstanding load, suggesting that biologic bone bonding may play the central role. The discrepancy between the BIC measurement and the mechanical withstanding load assay suggests that while bone formation around the implant must be a prerequisite, the development of osseointegration may rely on the actual bonding between the bone and the implant surface.
For many years, the existence of a thin layer of tissue between the bone and the implant surface has been reported in electron microscopy observations. This tissue layer is generally described as comprising an electron-dense zone 20 to 50 nm thick 9, 41and a 100- to 200-nm–thick zone without typical collagen fibers, 42followed by the collagen-rich bone tissue. However, considerable structural variations of this interface tissue have been pointed out, possibly due in part to sample preparation artifacts. Davies 43proposed that the electron-dense layer might be comprised of “globular accretions” that are highly mineralized. Cross sections of globular accretions may result in the reported variation in thickness of the interface tissue layer or so-called “cement line” 44( Fig 2-7). A study using a titanium-coated polystyrene cell culture plate revealed a globular accretion–like electron-dense structure abutting the titanium layer. 44The globular accretion–like interface layer was found to contain crystalline calcium phosphates similar to HA and the previously unreported thin collagen fibers. The precise molecular composition of the interface tissue has not been elucidated. However, it is postulated that molecules comprising the interface tissue between bone and the implant surface should hold the key to the mechanical withstanding force of osseointegrated implants.
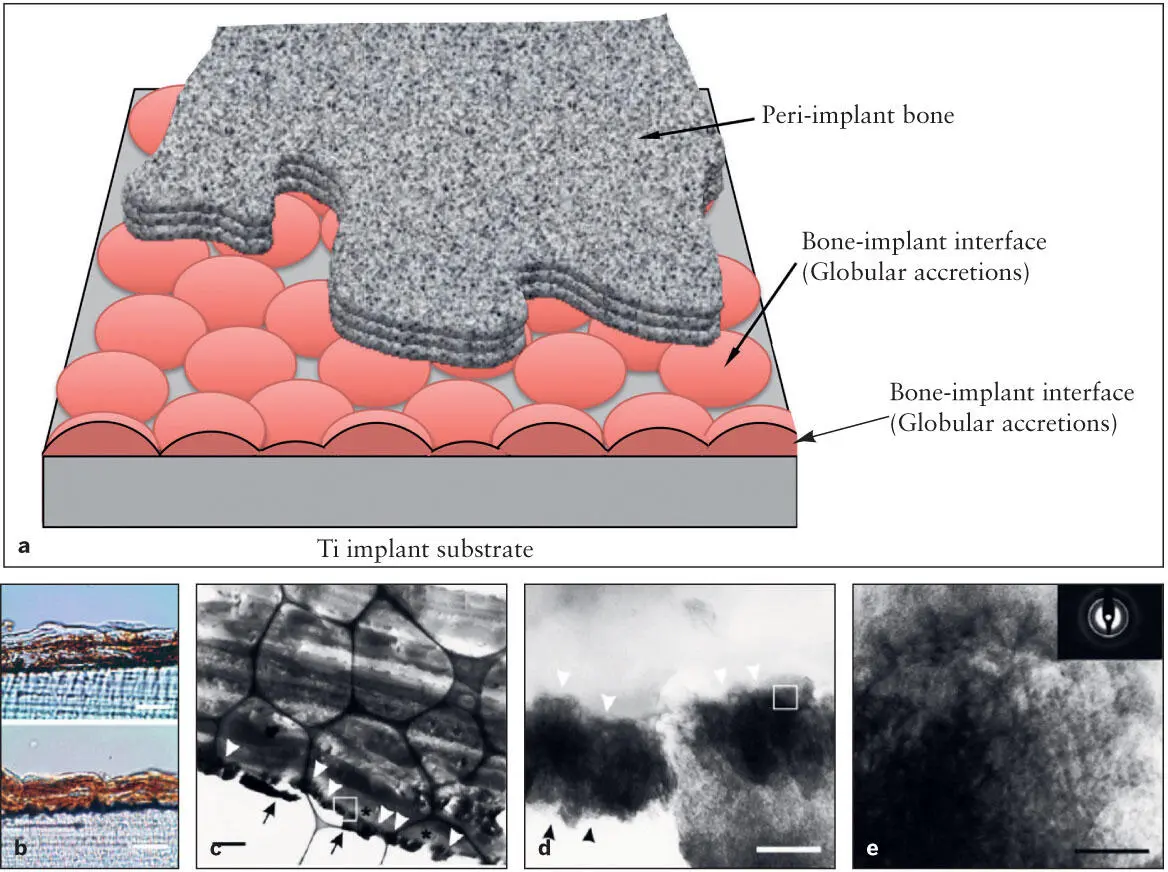
Fig 2-7 (a) Diagram of the implant-bone interface. There is a thin layer of interface zone between the peri-implant bone and the implant surface, which is thought to be composed of globular accretions. The cross section of a cluster of globular accretions may be equivalent to the zone of tissue of the cement line. It has been proposed that the molecular composition of this interface structure plays a key role in the function of osseointegration. (b) A recent in vitro study revealed that the osteogenic cells precipitated more mineralized tissue on the titanium-coated polystyrene cell culture plate (bottom) than on the control polystyrene surface (top) . (c) Transmission electron microscopy suggested an electron-dense zone of globular accretions (white arrowheads) on the titanium coating (arrows) . The globular accretion–like structures were interposed between the titanium coating and poorly mineralized bone (*). (d) A high magnification of the square in part c demonstrated the mineral content (arrowheads) as well as thin fibrous structures. (e) A close-up of the square in part d . The mineral content showed a crystalline structure consistent with HA. (Reprinted from Saruwatari et al 44with permission.)
It has been reported that this interface zone contains proteoglycans (PGs), 45although the amount of PGs has been debated. 46, 47PGs are associated with glycosaminoglycan (GAG) side chains, which provide a sticky consistency, and therefore it has been postulated that PG-GAG in the interface zone may play a role in the bonding between bone and implant. The adhesion of in vitro mineralized tissue to a titanium disk was moderately attenuated by the treatment of GAG degrading enzymes such as chondroitinase AC, chondroitinase B, and keratinase. 48Although this study suggested a functional role of PG-GAG for bone adhesion to the implant surface, the impact of chemical degradation of PG-GAG was surprisingly small. Therefore, the shear strength of osseointegrated implants to withstand occlusal load appears to involve more complex mechanisms.
The interface tissue (also known as the cement line ) contains osteopontin (OPN). 49OPN is a noncollagenous ECM molecule in bone. It has an integrin-binding sequence, suggesting cell adhesion functions. In addition, because OPN has been found in high levels in mineralized tissue of bone and teeth, its postulated functions include regulation of bone remodeling. However, genetically modified mice lacking OPN were surprisingly normal, and their skeletal tissues developed without any complications. 50The cement line of OPN-deficient mice was also found to exhibit the normal structure. Recently, a reevaluation of OPN-deficient mouse bone revealed that there was a 30% decrease in bone fracture toughness, while the bone mass remained unaffected. 51The nanoindentation assay showed that the stiffness, not the hardness, was significantly decreased. Although this conclusion is highly speculative, the high OPN content in the cement line may contribute to the increase in stiffness of the mineralized interface tissue between the bone and the implant surface, which could contribute to an increase in mechanical withstanding shear strength.
Читать дальше