Controlled nanostructuring of titanium surfaces experimentally used to optimize nano-size
A recent study described a new method to create a uniform nanonodular surface topography on titanium implants. This method utilizes a newly discovered phenomenon of titanium nanonodular self-assembly during physical vapor or sputter deposition of titanium onto specially conditioned micro-rough titanium surfaces. 80The size of nanonodules can be controlled by altering the deposition time. The newly added nanostructures must be smaller than the existing microrough configurations of the implant surface ( Fig 2-12). 79Using this self-assembly method, implants with acid-etched surfaces were converted to micro/nano-hybrid surface topographies ranging from 100 to 1,000 nm in diameter ( Fig 2-13). 79The nanostructure creation effectively creates geometric undercuts and increases the surface area by up to 40% compared with the acid-etched surface with microrough surface topography.
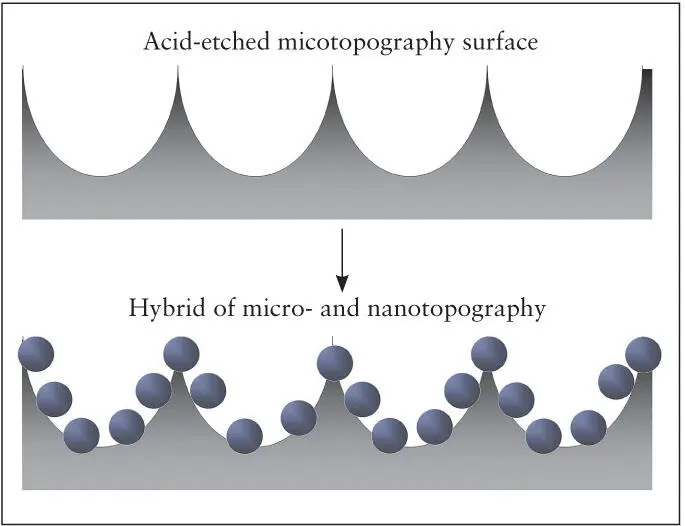
Fig 2-12 A method has been developed for fabricating micro/nano- hybrid titanium surface topography. This increases surface area, creates mechanical undercuts, and maintains the existing microrough surface topography. (Reprinted from Moy et al 79with permission.)
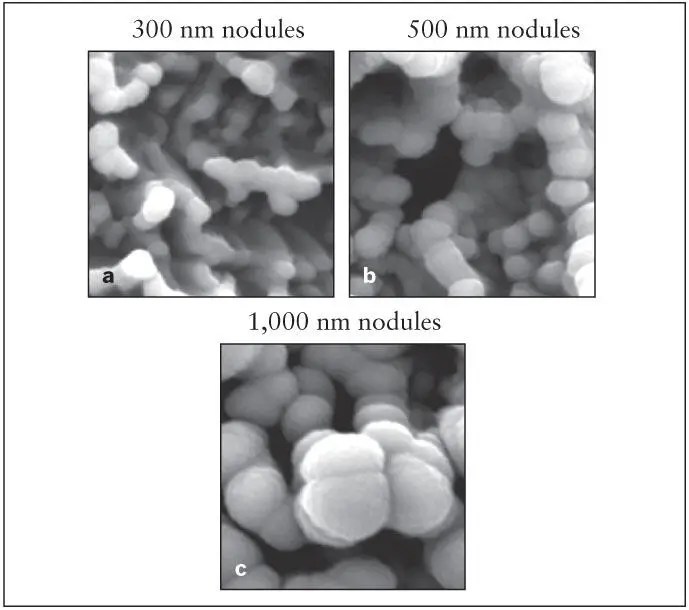
Fig 2-13 (a to c) Three different sizes of nanonodules of titanium created on the acid-etched microrough titanium surfaces using a nanonodular self-assembly method. (Reprinted from Moy et al 79with permission.)
The surface topography created with this method closely resembles the surface morphology of biomineralized bone matrices. 81This nanoenhanced implant surface selectively promoted function of cultured osteoblasts but not fibroblasts. Implants with microrough surface topography promote and accelerate differentiation of osteoblasts but inhibit their proliferation. Implants prepared with microrough surfaces enhanced with nano-sized nodules substantially enhanced both of these cell activities. These biologic effects were most pronounced when the nano- nodules were 300 nm in diameter. An implant biomechanical test in a rat femur model revealed that the strength of bone-titanium integration was more than three times greater for the implants with the microrough surface enhanced with nanonodules compared to the implants with unenhanced microrough (acid-etched) surfaces. These results suggest that the establishment of uniquely functionalized nano-in- micro titanium surfaces improved osteoconductivity and may provide a biomimetic micro-to-nano-scale hierarchical model to optimize the nanofeatures of dental implant surfaces and other biomaterials.
Biologic Aging and Photofunctionalization of Implants
Many implants are packaged in plastic and are then sterilized with gamma radiation. This process contaminates the implant surface with hydrocarbons and other carbon-containing impurities. Bioreactivity of the implant surface is impaired, the surface charge is changed from positive to negative, and the surface becomes less wettable. As a result, adsorption of plasma proteins, platelet activation, and recruitment and attachment of osteogenic cells are inhibited.
Biologic aging of implant surfaces
A series of recent studies reported significant changes in the osteoconductivity and other biologic capabilities of titanium implant surfaces over time. These studies have indicated that the bioreactivity of titanium implant surfaces degrade as a function of time, and this phenomenon has been referred to the biologic aging of titanium . 82– 86This time-dependent degradation can be substantial, as the strength of osseointegration measured by a biomechanical implant push-in test model can be reduced by 50% for aged titanium surfaces compared to newly prepared titanium surfaces. Moreover, a BIC area higher than 90% can be obtained for new, uncontaminated titanium surfaces compared to a BIC area less than 60% for aged surfaces. The degradation of the implant surface bioreactivity appears to be primarily associated with the reduced capability of aged titanium surfaces to adsorb plasma proteins, activate platelets, and attract osteogenic cells. 82– 86
Surface property changes associated with the biologic aging of titanium
An analysis of surface chemistry using x-ray photoelectron microscopy has demonstrated that the percentage of carbon molecules on titanium surfaces increases with time. 82, 87The percentage of carbon, which in one study was found to be 14% on the acid-etched titanium when first prepared, increases to 63% after 4 weeks of storage under ambient conditions. 82More than half of the titanium surface does not appear as titanium at the molecular level. The increase of surface carbon is due to the deposition of carbon-containing impurities from the local environment onto titanium surfaces, consisting primarily of hydrocarbons. Significantly, the capability of titanium surfaces to attract proteins and osteogenic cells has been shown to have a strong inverse correlation with the percentage of surface carbon. This data implies that the presence of surface carbon plays a crucial role in determining bioreactivity of titanium implant surfaces. 82
Implants with hydrophilic surfaces are more bioreactive. However, the contamination of the implant surface described previously degrades the hydrophilicity of titanium implant surfaces and impairs its ability to adsorb plasma proteins. Recent studies have shown that titanium surfaces, immediately after preparation, regardless of the types of processing used, display a water contact angle ranging from 0 to 5 degrees. Such surfaces are considered superhydrophilic. However, the superhydrophilic nature of freshly prepared implants gradually attenuates, and after 1 week the surface becomes hydrophobic and the contact angle increases to over 40 degrees. The contact angle for 4-week-old acid-etched implant surfaces increases to 60 degrees and has been found to be as high as 90 degrees 82, 83, 87– 90( Fig 2-14).
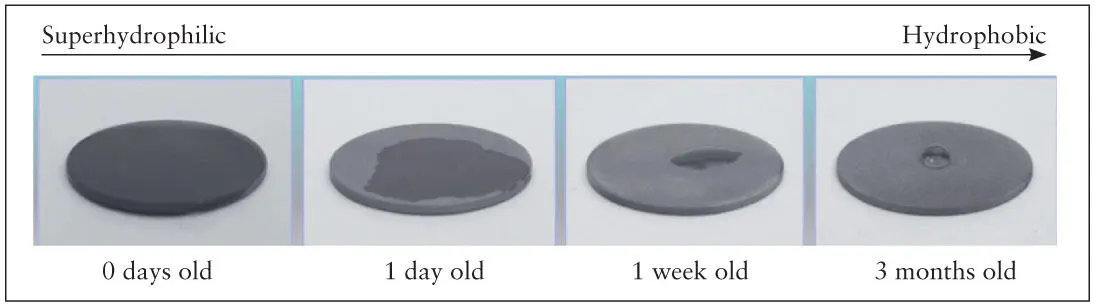
Fig 2-14 Time-dependent changes in hydrophilic nature of titanium surfaces. Titanium surfaces are superhydrophilic when prepared but become hydrophobic after 1 week, and the degree of hydrophobicity increases with time. (Reprinted from Moy et al 79with permission).
Photofunctionalization
In response to the degradation of the bioreactivity of titanium implant surfaces as a function of time, recent research efforts have focused on developing a means of removing these contaminants and restoring implant surfaces to their previous level of bioreactivity prior to surgical placement. Studies to date indicate that carbon contamination of titanium implant surfaces occurs regardless of the method used to create the microsurface topography. The method described in this section has been shown to be effective in decontaminating all of the titanium implants tested.
Photofunctionalization is defined as a phenomenon whereby the physicochemical properties and bioreactivity of titanium implant surfaces are restored after UV light treatment. 84, 90– 95Titanium surfaces that have aged (ie, older than 1 month after preparing the surface) become hydrophobic. The contact angle of a droplet of water applied to the surface is generally above 60 degrees and often closer to or above 90 degrees on most surface types. This loss of wettability is common for all surface topographies of titanium. 82, 87, 88, 90, 91, 93, 95, 96When water droplets are applied to the surfaces of these implants, the droplet retains its hemispheric form. After treating these titanium implant surfaces with UV light, the surfaces regain their superhydrophilicity, and the contact angle is reduced to almost zero (see Fig 2-15).
Читать дальше