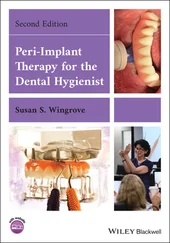
Fig 2-15 (a to c) Aged untreated implant. (d to f) Implant after photofunctionalization. Photofunctionalization improves the hemophilicity of the implant surface (part a vs part d ). Photofunctionalization enhances cell adsorption of plasma proteins, enhances attraction of mesenchymal stem cells, and promotes and accelerates differentiation of mesenchymal stem cells into osteoblasts and enhances cell spreading. Note the contrast between part b and part e . The result is that the BIC area is increased to almost 100% in a rat model. Note the difference between part c (untreated surface) and part f (treated surface). (Reprinted from Moy et al 79with permission.)
As mentioned previously, soon after the implants are prepared, hydrocarbons begin to accumulate on their surfaces, most likely from the atmosphere and the surrounding environments during surface preparation and storage. 17, 37, 82, 87, 95– 103UV treatment reduces the carbon percentage to less than 20% depending upon the conditions. 95, 96, 104Titanium surfaces covered by hydrocarbons are known to be negatively charged, which makes the implant surface repellent to plasma proteins and osteoprogenitor cells, which are negatively charged. 83, 84, 86, 94After photofunctionalization, titanium surfaces are converted to electropositive and serve to attract plasma proteins and osteoprogenitor cells. 84, 86, 94, 105
The biologic effects of photofunctionalization are shown in Fig 2-15. Following photofunctionalization of aged implants, the strength required to separate the investing bone from the implant surfaces—as measured by a biomechanical implant push-in test model—is increased by a factor of three. 95Moreover, BIC area has been shown to increase to above 90% and in some specimens to almost 100%. In contrast, the BIC area for untreated implants is generally in the range of 50% to 60%. 95, 96Photofunctionalization has been shown to be effective for all surface topographies tested (anodized, dual acid etched, sandblasted/acid etched, machined surface). 88, 93, 96, 104, 106, 107
In summary, in vitro studies conducted to date have demonstrated that photofunctionalizing titanium implants (1) increases adsorption of plasma proteins on the implant surface, (2) facilitates attachment of and retention of osteogenic cells to the implant surface, (3) facilitates spread of osteoblasts on the implant surface, (4) increases cell proliferation, and (5) promotes and accelerates differentiation of mesenchymal stem cells once they migrate to and bind to the surface of the implant. 88, 93, 96, 104, 106, 107
Clinical implications
To date, most efforts to improve the osteoconductivity of osseointegrated implants have been focused on changing the micro- or nanosurface morphology and chemistry. However, the studies cited earlier have shown that the osteoconductivity of modern implant surfaces can be dramatically enhanced by ridding the implant surface of carbon contaminants. If the degree of BIC established by photofunctionalized implants can be sustained once the implants have been placed under function, these studies imply that several difficult clinical challenges can be addressed. Shorter implants may be employed than previously have been shown to be feasible with untreated surfaces, and implants placed in poor-quality bone sites may be more predictable regardless of whether initial stabilization is accomplished.
A chairside device has been developed for commercial use that emits UV light of multiple wavelengths. The implants are photofunctionalized for 15 minutes before use in patients. Several authors have reported their initial experiences. 108– 112Initial reports are promising, but long-term studies are needed to clarify the degree of advantage offered by chairside photofunctionalization.
Other applications
Photofunctionalization appears to have application beyond dental implants. Photofunctionalized titanium mesh, used to house and contain bone grafts, has been shown to be more osteoconductive and able to facilitate more bone regeneration in animal models 113and humans. 110Photofunctionalized orthodontic miniscrews have been shown to develop improved bone anchorage and better resist lateral forces. 114Photofunctionalization is also being employed with increasing frequency in orthopedic surgery.
Degradation of Titanium Dental Implant Surfaces
Dental implants are most commonly made from commercially pure titanium (cpTi) grade II or grade IV. Grade IV has higher strength and lower corrosion resistance than grade II. 115The abutment and prosthetic components are made of titanium alloy (Ti6Al4V) due to its high tensile strength; however, the corrosion resistance of Ti6Al4V is lower than cpTi. 116
After surgical implant placement and prosthetic restoration, the dental implant is susceptible to biochemical degradation. Recent studies on the degradation of dental implants and prosthetic components in the presence of microorganisms and the corrosive environment of the oral cavity have gained more attention in the recent literature. 117– 119Chemical (dry) and electrochemical (wet) corrosion causes different forms of degradation, which can occur in the oral cavity. Several studies 118– 125have shown particles derived from dental implants in peri-implant tissues. It has been suggested that these particles are released from dental implants due to nontherapeutic and therapeutic causes. The released titanium micro- and nanoparticles are cytotoxic 126and act as foreign bodies to the immune system. As a consequence, the released particles activate osteoclasts and local inflammatory cells and increase the expression of pro-inflammatory cytokines such as tumor necrosis factor α (TNF-α), which trigger an inflammatory cascade in the peri-implant tissues. 119, 125
Nontherapeutic causes
Nontherapeutic causes of implant surface degradation include the presence of biofilm and inflammatory responses (ie, peri-implant diseases), wear from micromovement of contacting surfaces at the implant-abutment connection, 127or the detachment of particles from titanium implant surfaces during insertion. 120The latter is related to the corrosive effects of therapeutic agents such as citric acid, bleaching solutions, and fluoride, 118, 119or surgical treatment of peri-implant diseases when implantoplasty is carried out to smoothen the implant surface. 123, 128
The formation of an oral biofilm can decrease the pH of the oral environment and degrade the titanium surface due to the presence of corrosive metabolites such as the production of lactic acid by Streptococcus mutans in the presence of sucrose. 129Occlusal loading can cause micromovements of the contacting surfaces at the implant-abutment connections. The combined wear and corrosion from the two contacting surfaces in the presence of a biofilm is termed tribocorrosion and can lead to degradation of the titanium implant surface into the surrounding peri-implant tissues. 127, 130The mechanical wear facilitates corrosion by damaging the passive protective layer of TiO 2and exposing the implant surface to corrosion. The corroded implant surface becomes more vulnerable to further mechanical wear. Tribocorrosion is also intensified by the use of rough-surfaced dental implants, which are more susceptible to biofilm accumulation compared with smooth-surfaced implants. 131Numerous studies on surface deterioration of orthopedic implants suggest that aseptic loosening of the implant is related to the production of wear debris from the prostheses. 132, 133
Therapeutic causes
The most important steps in the treatment of peri-implant diseases are debridement and decontamination of the implant surface. Both debridement and decontamination can be achieved by chemical and mechanical means or a combination of both. 134Such means remove the biofilm and inflammatory tissue, but at the same time they can affect the implant surface and connection.
Читать дальше