Besides the injured collagen fibers and tissues, biomaterials placed in the body can activate platelets at different rates. Platelets are considered to be the first cell-like structures to adhere to the implant, and they immediately start secreting bioactive factors and organizing the fibrin clot. It takes only 2 minutes to initiate the fibrin clot formation on titanium surfaces. 7Platelet adhesion and activation on different biomaterials and material surfaces have become subject to intense investigation because the resulting fibrin clot scaffold is thought to determine inflammation behavior and subsequent wound healing around the biomaterial.
Hong et al 8reported that there was much less platelet activation on the surface of stainless steel plates than on titanium plates. When used as an endosseous implant, stainless steel is surrounded by a sustained inflammatory reaction, resulting in minimal, if any, direct bone contact. 9Therefore, the ability to activate platelets and form the fibrin clot may be an important first step in osseointegration.
Effect of implant surface modifications on fibrin clot formation
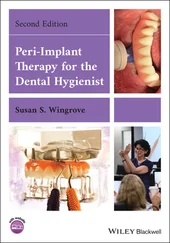
Recent research and development efforts have been directed toward creating more bioactive titanium surfaces suitable for increased platelet adhesion. Moderately rough surface topography has been shown to increase platelet activation prepared by various methods: double acid etching 10( Fig 2-1), fluoride ion–modified grit blasting, 11sandblasting, and acid etching. 12
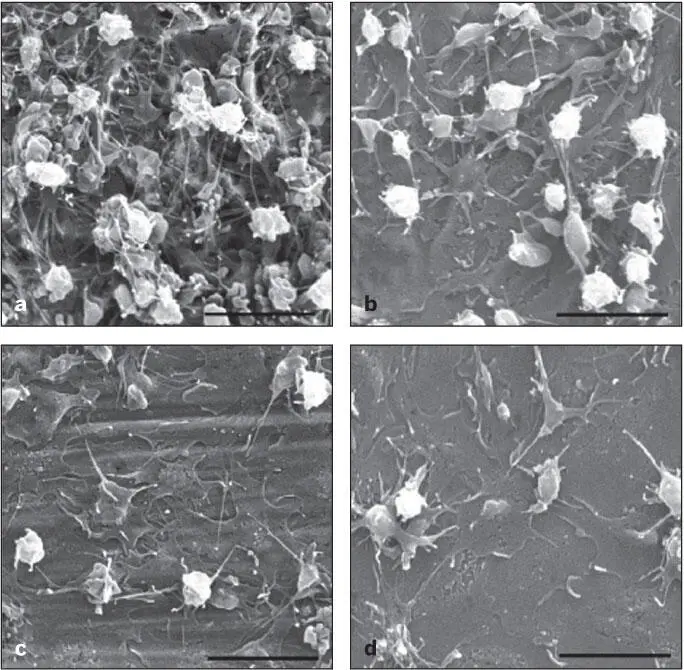
Fig 2-1 Scanning electron micrographs (SEMs) of platelet-rich plasma contact (for 30 minutes) with commercially pure titanium: (a) double acid etched; (b) 320-grit abraded; (c) machined; (d) polished. The platelet aggregation and fibrin clot formation were more significant on roughened titanium surfaces. (Reprinted from Park et al 10with permission.)
Interestingly, in the field of vascular stent development, research efforts have been directed toward decreasing the adhesion of platelets and thus minimizing thrombosis formation. In fact, the micrometer to nanometer surface topography created on the titanium vascular stent 13or polymer materials 14was shown to decrease the platelet adhesion. The stark contrast in the observations regarding endosseous implants and vascular stents that both carry moderately rough titanium surface topography may suggest that not only the surface roughness but also other factors might determine the initial host response.
Complex surface topography is generally associated with increased hydrophobicity, which prevents the adhesion of platelets and cells. Acid etching used to create microtopography increases the surface precipitation of titanium dioxide, or titania (TiO 2), 10whereas alkali treatment results in the formation of charged TiO 2on the titanium surface. 15These surface modifications involving TiO 2have been postulated to control platelet adhesion and activation. TiO 2is a stable and relatively bioinert material that is largely responsible for the biocompatibility of titanium implants. However, the therapeutic role of TiO 2has not been well characterized. The zeta potential or electron charge of the surface of TiO 2is influenced by pH levels and the presence of various ions such as Ca 2+. Both acidic (low pH) and alkali (high pH) treatments are known to change the zeta potential of TiO 2, contributing to the modulated cell and protein adhesion behavior. Recent studies suggest that the proprietary SLActive preparation (Straumann) or postfabrication ultraviolet (UV) light treatments could increase surface hydrophilicity or surface charge of titanium implants. Characterization of their effect on the platelet behavior and fibrin clot formation has just begun, 16which may present an important clue to understanding the role of surface reactivity and zeta potential on osseointegration.
It must be noted that hydroxyapatite (HA) surfaces show somewhat different platelet adhesion and activation properties as compared with titanium surfaces. The HA surface disproportionately increases complement activation in the fibrin clot 11and increases adsorption of serum proteins. 17Therefore, new surface modifications employing a hybrid of TiO 2and HA 18– 22may present a unique opportunity to expand the available armamentaria for better optimization of platelet activation and fibrin clot formation relevant to osseointegration.
Platelet activation occurs at the tissue injury site and on the surface of biomaterials. However, the tissue injury site activates fibrin clot formation much more efficiently than do titanium materials. 12Experimentally, the periodontal ligament on the freshly extracted tooth induced significantly more active clot formation than other artificial materials tested. 23Therefore, there may be a gradient of fibrin clot network around the implant that is more organized and matured on the osteotomy-wounded bone surface than on the implant surface 7, 23( Fig 2-2).
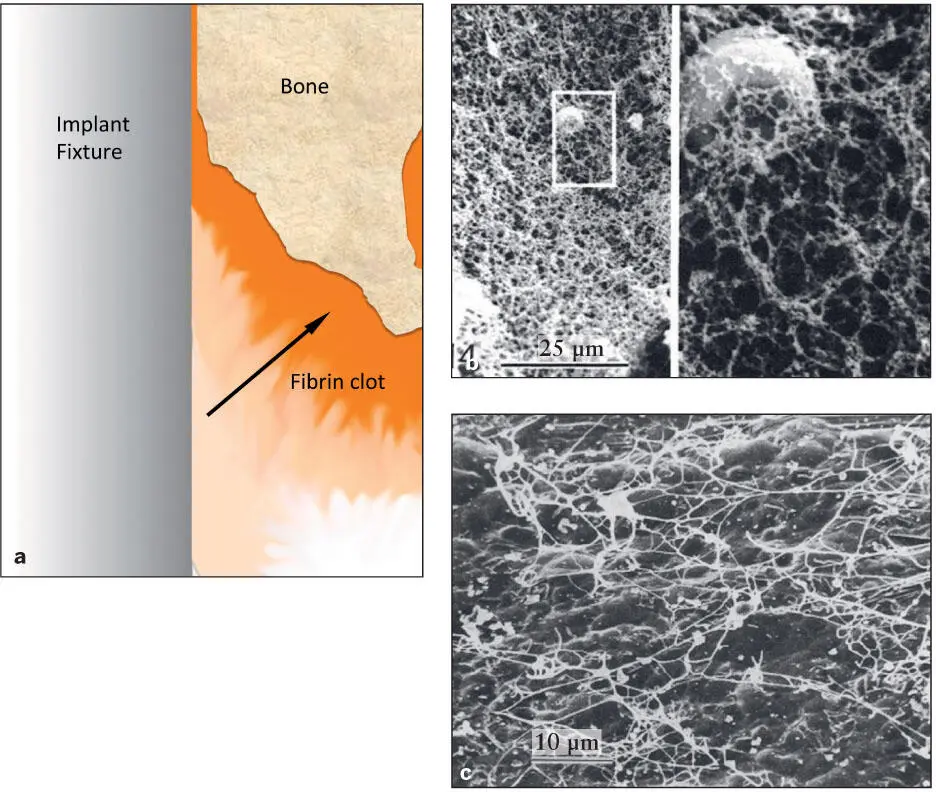
Fig 2-2 (a) Diagram of fibrin clot organization around an implant immediately after placement in the osteotomy site. Platelet activation is significantly more efficient on the exposed collagen from the injured tissue than on the titanium surface. As a result, a gradient of fibrin clot (arrow) is organized from the implant surface to the bone surface. (b) A cleaned extracted human tooth with remaining periodontal ligament was dipped in a fresh extraction socket for 60 seconds, and the surface was examined by SEM. A dense fibrin clot was already formed and organized (magnification: left , ×880; right , ×4,400). (Reprinted from Steinberg and Willey 23with permission.) (c) A similar experiment was performed with a titanium plate. A titanium plate was dipped in a fresh extraction socket for 60 seconds. The fibrin clot formed a different architecture. (Reprinted from Steinberg et al 7with permission.)
Fibrin Remodeling (Days to Weeks) and Bone Formation (Weeks) to Bone Remodeling (Years)
Fibrin scaffold network and macrophage infiltration
The wound-induced fibrin clot formation results in the organization of a fibrin scaffold network necessary for the succeeding tissue repair. Although the structure of fibrin networks is determined by multiple factors such as pH, clotting rate, and coagulation factor concentrations, polymerization of fibrin molecules generally occurs within the first 24 hours of wounding. The organized fibrin network is further modified by the incorporation of fibronectin molecules, which serve as the critical factor influencing bone formation in the fibrin scaffold. A recent study suggested the presence of macrophages within the fibrin clot adjacent to a dental implant within 12 to 24 hours. 24The early and transient expression of C-X-C chemokine receptor type 4 (CXCR4; a cell surface receptor of monocytes/macrophages) in this study supports the involvement of macrophages in the process of osseointegration as well as the process of clearing the tissue debris ( Fig 2-3). 25
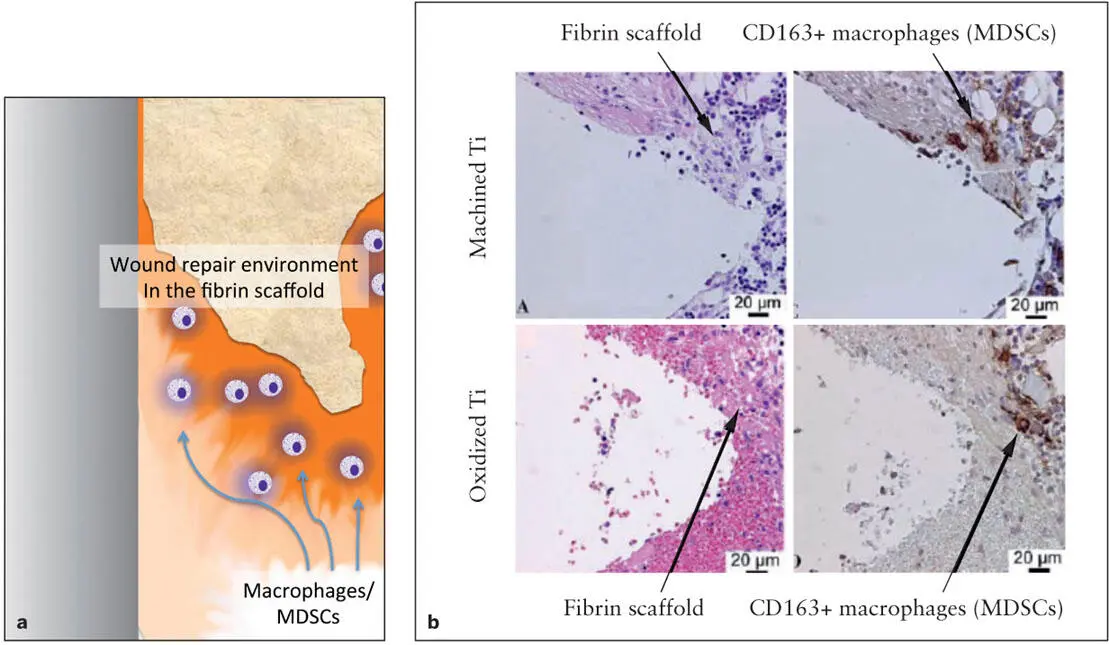
Fig 2-3 A diagram of bone formation around an implant. (a) Immediately after the fibrin clot scaffold is formed, bone marrow–derived myeloid cells called myeloid-derived suppressor cells (MDSCs) migrate into the mature fibrin clot and organize the local environment for wound repair. MDSCs stimulate new vascular formation and suppress wound-induced inflammation. (b) After 24 hours of implantation, the fibrin clot scaffold is already organized on the implant surface. Immunohistologic evaluation revealed the infiltration of CD163+ macrophages (or MDSCs) stained in brown in the fibrin scaffold. (Reprinted from Omar et al 25with permission.)
Читать дальше