Macrophages are classically described as pro-inflammatory phagocytic cells (M1 macrophages) that clear tissue debris and eliminate bacterial infection. It has been demonstrated that there are alternative differentiation pathways generating M2 macrophages that are capable of resolving inflammation and actively inducing angiogenesis for tissue repair. 24It must be noted that the study by Omar et al 25further suggested that macrophages infiltrating the fibrin scaffold around the implant were recognized by the CD163 cell surface marker. A subset of macrophages carrying CD163 are thought to express the M2 phenotype and are considered myeloid- derived suppressor cells (MDSCs). MDSCs originate in bone marrow and resolve inflammatory reactions by suppressing T-cell activities. In addition, MDSCs induce angiogenesis and secrete a set of growth factors that support rapid wound healing. 26Therefore, the presence of macrophages and MDSCs may be critical for establishing a tissue repair environment for wound healing and bone formation.
Distance and contact osteogenesis
As seen in wound healing following tooth extraction, initial bone formation occurs in the bottom of the socket, suggesting the establishment of a tissue repair environment in the mature fibrin network ( Fig 2-4). Fibronectin is a large glycoprotein with active binding sites not only to fibrin but also to other extracellular matrix (ECM) molecules and integrin-expressing cells. Incorporation of fibronectin in the fibrin network has been shown to be important for supporting macrophage function. The earliest bone formation should occur in the matured fibrin network adjacent to the osteotomy-exposed alveolar bone. An experimental implant model in mice demonstrated the early sequence of bone formation within the well-organized fibrin network that was more apparent on the bone surface. 27This study further demonstrated the highly localized fibronectin molecules associated with the bone surface fibrin network. Bone tissue formation away from the implant is called distance osteogenesis , 28which involves an ordinary sequence of bone wound healing as often seen in the tooth extraction socket or in the bone marrow ablation site.
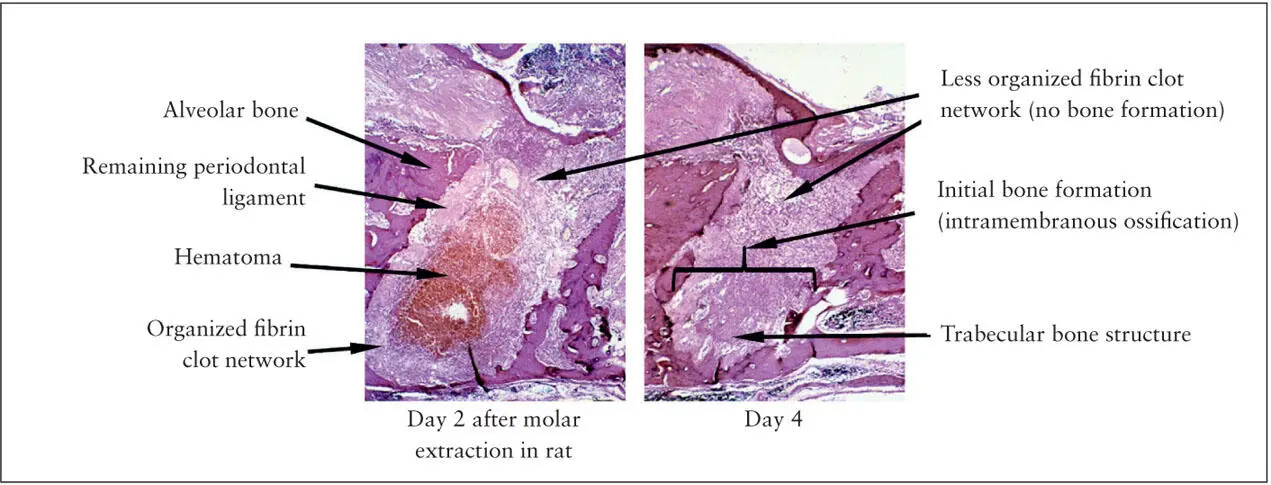
Fig 2-4 After rat molar extraction, the fibrin clot is organized at the bottom of the extraction socket (left) . The bone remodeling first occurs within the fibrin clot scaffold (right) . The cervical region where the initial fibrin clot formed is less organized.
During this period, the implant surface is still associated with a less organized fibrin scaffold network. However, the implant surface fibrin network is rapidly remodeled with the incorporation of fibronectin and provides the scaffold for bone formation. Distance osteogenesis may now approach in close proximity to the implant surface, while the new bone formation can occur within the now-matured fibrin network surrounding the implant. Contact osteogenesis describes this bone formation near the implant surface, which may be significantly affected by the different environment influenced by the implant material. 28The gap between regenerating bone and the implant surface may be completely filled as early as 7 days after surgery, establishing the histologic osseointegration.
Fibrin clot formation and remodeling take place rapidly at the tissue injury site, where distance osteogenesis should be initiated immediately. Slow fibrin network maturation on the implant surface may cause delayed bone formation. In other words, contact osteogenesis around the implant occurs in a sequence, and the bone-to-implant contact (BIC) is established during the last stage of bone remodeling 27( Fig 2-5). There is a small but distinct time lag between distance osteogenesis and contact osteogenesis. However, active implant surface modifications may significantly accelerate contact osteogenesis.
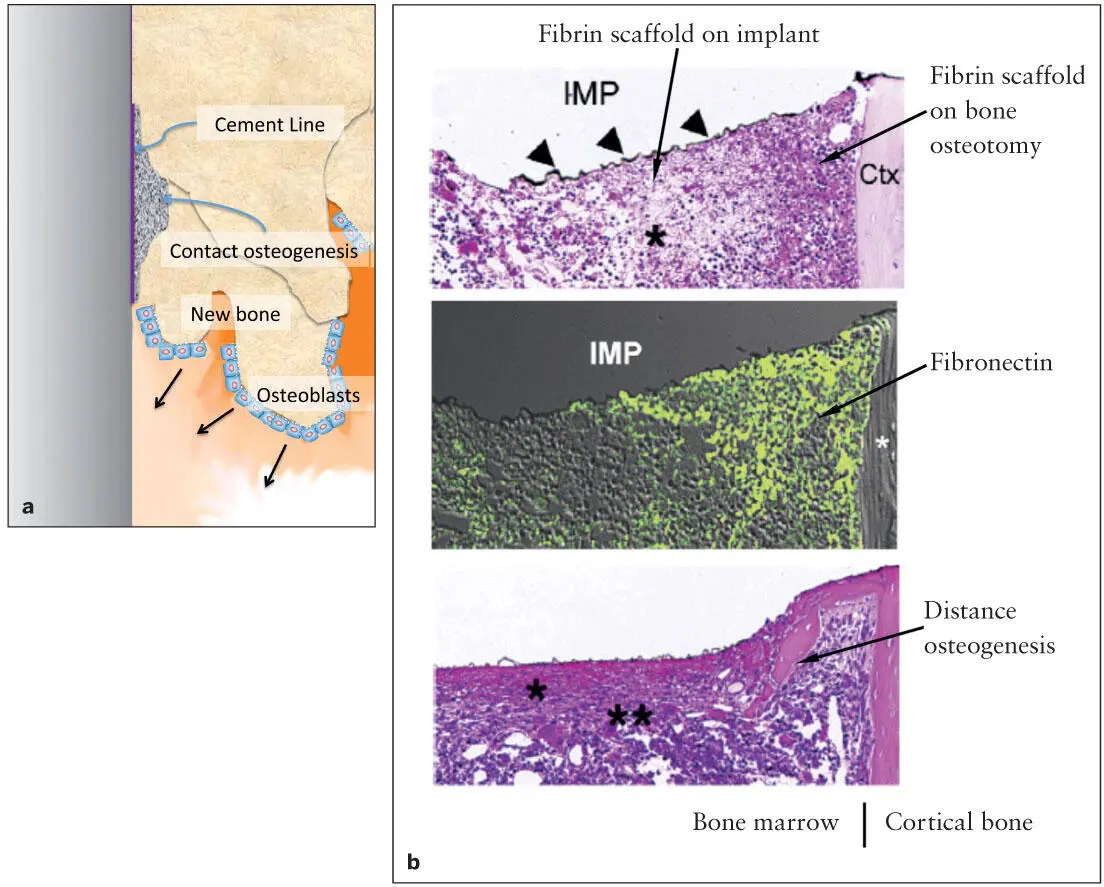
Fig 2-5 (a) The first bone formation occurs within the fibrin scaffold associated with the bone tissue exposed by osteotomy. Along with the delayed organization of the fibrin scaffold on the implant surface, bone formation catches up and eventually establishes BIC. (b) An experimental implant (IMP: titanium-coated [arrowheads] plastic implant) was placed in an osteotomy site of a mouse femur. Fibrin clots were organized 1 day after implant placement (top) . The fibrin scaffold associated with the bone osteotomy site and cortical bone (*) appeared to be more organized than that on the implant surface. Fibronectin (green) was found in the organized fibrin scaffold close to the bone osteotomy site (middle) . Two days after implant placement, the initial bone formation was detected within the organized fibrin clot containing fibronectin, while the fibrin network (*) on the implant surface appeared to be still immature. (Reprinted from Jimbo et al 27with permission.)
Characteristics of Peri-implant Bone
Peri-implant bone, which is formed in close proximity to the implant surface, plays a central role in the sustained support of the implant. Peri-implant bone is formed within the fibrin scaffold surrounding the implant and is likely to be influenced by the implant surface topography, chemistry, and charged energy. These factors may affect the unique characteristics of the bone deposited onto the surface of the implant, which could directly or indirectly contribute to the maintenance of osseointegration. This section discusses the biomechanical characteristics, the shear strength at the bone-implant interface, and the long-term stability of peri-implant bone.
Biomechanical characteristics of peri-implant bone
Ideally, the intrinsic biomechanical properties of peri- implant bone should be capable of withstanding functional forces. It has been shown that hardness and stiffness of peri-implant bone may be associated with certain implant surface modifications. Butz et al 29employed nanoindentation assays to measure the hardness and Young modulus of peri- implant bone associated with a relatively smooth machined or double acid-etched titanium implant in a rat model. The hardness of peri-implant bone associated with a relatively smooth (machined) implant was progressively increased from 2 weeks to 4 weeks after the surgical implant placement and reached the equivalent hardness of trabecular bone. The bone hardness associated with a moderately rough (double acid-etched) implant similarly underwent the progressive increase; ultimately, however, it was found to be much harder and reached the equivalent hardness of cortical bone. Recently, a similar experiment in a rabbit model revealed that the hardness of peri-implant bone almost doubled when a moderately rough (sandblasted/acid-etched) implant surface was further modified with a nano-HA coating. 30
Once osseointegration is established, the intrinsic biomechanical properties of peri-implant bone should greatly contribute to the load-bearing function. It is intriguing that peri-implant bone may reach the hardness of cortical bone around implants with moderately rough and more complex surfaces. The primary mechanism determining the bone hardness and stiffness has been debated. A positive correlation between the stiffness and bone mineral density was demonstrated in bovine cortical bone 31and porcine mandibular condyles. 32
Bone is a composite tissue of collagen-based fibers and crystalline HA. The bone mineral content is regulated by the organic collagen matrix, which is largely composed of type I collagen. Fragile bone is the primary phenotype of a group of genetic disorders called osteogenesis imperfecta . Patients with these disorders experience bone fractures even during normal physical activity. A number of mutations have been discovered in type I collagen genes; however, the most severe form of osteogenesis imperfecta is associated with the genetic mutations in enzymes that control collagen cross-linking, such as prolyl-3-hydroxylase (P3H) 33and cartilage-associated protein (CRTAP). 34In addition, prolyl-4-hydroxylase (P4H) is also involved in collagen cross-linking, and collectively these enzymes are critical in determining the intrinsic bone mechanical properties. In vitro biomimetic mineralization on collagen films using a polymer-induced liquid-precursor mineralization process further supports the notion that increased collagen cross-linking significantly stimulates mineralization and increased intrinsic mechanical properties. 35
Читать дальше