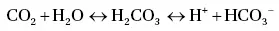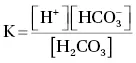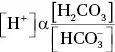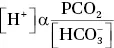Normal values are listed in Table 3.1.
A sample of arterial bloodmay be obtained from any artery, but the radial arteryat the wrist and the brachial arteryin the antecubital fossa are the sites most commonly used. The blood enters the heparinised needle and syringe under its own pressure with a pulsatile action. The syringe containing the arterial blood is capped, placed in iceand analysed in the laboratory within 30 minutes of sampling.
Review of acid/base balance
CO 2dissolves in H 2O and forms carbonic acid (H 2CO 3), which dissociates into H +and HCO 3 –in a constant relationship:
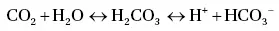
Table 3.1 Normal values for arterial blood gases whilst breathing normal room air at sea level
| pH |
7.35–7.45 |
| PCO 2 |
4.5–6.0 kPa, 34–45 mmHg |
| PO 2 |
11–14 kPa, 83–105 mmHg |
| Actual bicarbonate (aHCO 3 – ) |
22–26 mmol/L |
| Standard bicarbonate (sHCO 3 – ) |
22–26 mmol/L |
| Base excess |
–2 to +2 mmol/L |
| Oxygen saturation |
96–98% |
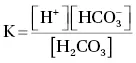
Thus:
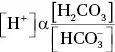
As [H 2CO 3] directly relates to the partial pressure of CO 2:
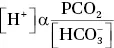
In other words, for a given concentration of bicarbonate, PCO 2has a direct linear relationship with [H +] (and thus an inverse relationship with pH, which is the negative logarithm of [H +]).
Similarly, for a given PCO 2, there is a direct relationship between [HCO 3 –] and pH.
These relationships can be represented graphically ( Fig. 3.9).
Bicarbonate concentration
Most blood gas analysers provide two different measurements of bicarbonate – ‘actual bicarbonate’ and ‘standard bicarbonate’ – in addition to another value, ‘base excess’. This can cause confusion, although it needn’t. The analyser measures the bicarbonate level in the blood sample. This actual measurement is (conveniently) known as the actual bicarbonate(aHCO 3 –). As can be seen in Fig. 3.9, the actual level is directly dependent on the PCO 2(for a given pH: the higher the PCO 2, the higher the aHCO 3 –; the lower the PCO 2, the lower the aHCO 3 –). What we’d like to know is what the bicarbonate would have been if the PCO 2had been normal (5.3 kPa) because that gives us a direct handle on ‘what the metabolic system is doing’. If we know the actual bicarbonate (aHCO 3 –) and the PCO 2then we can calculate that value. However, we don’t need to, the machine will do it for us. This calculated bicarbonate value for a ‘standard’ PCO 2is (conveniently) known as the standard bicarbonate(sHCO 3 –). Either aHCO 3 –or sHCO 3 –can be used in the interpretation of blood gases, but the sHCO 3 –takes out the immediate effect of CO 2on the bicarbonate level and can loosely be regarded as giving a more direct indication of the metabolic activity influencing acid/base balance, (I think it’s easier). The base excesstakes into account the fact that there are other buffers apart from bicarbonate in the blood. It tells a similar story to the bicarbonate level in terms of acid/base disturbance. Its principal advantage is the ease with which its normal range can be remembered. As one might anticipate from the name, the ‘excess’ should be zero (normal range is 0±2 mmol/L). There aren’t many numbers easier to remember than zero.
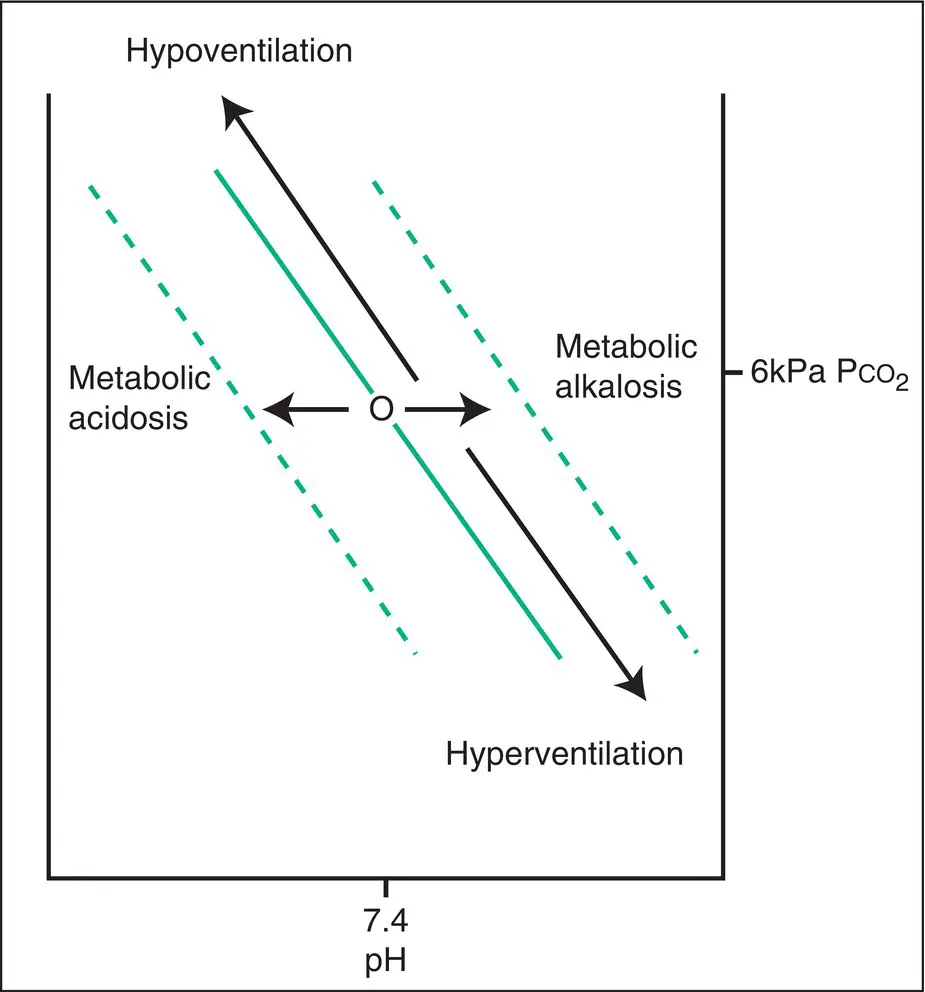
Figure 3.9 Bicarbonate isopleths (diagonal lines; the bicarbonate level is constant along the lines). It can be seen that, if the bicarbonate level and PCO 2are known, the pH can be calculated. Indeed, if any two of the three values of bicarbonate, pH and PCO 2are known then the other value can be calculated. These values are yoked together. A change in ventilation will move the arterial point up or down an isopleth as shown, changing pH (bicarbonate level does not change). A pure metabolic disturbance (before any respiratory response) changes the bicarbonate level, moving from one bicarbonate isopleth to another and changing pH.
The three variables pH, PCO 2and bicarbonate are yoked together as just described. Analysis of their values provides information on the acid/base balance of the body and the broad nature of its cause. It may also provide information about the chronicity of an abnormality.
Changes in the acid/base status caused by changes in PCO 2(hyper‐ or hypoventilation) are termed respiratory. Changes in acid/base status caused by changes in bicarbonate are termed metabolic. A disturbance in one system tends to prompt a compensatory response in the other. When needed, the respiratory system responds promptly, and changes are evident within seconds to minutes. The metabolic system, largely regulated via renal excretion, is much slower, taking between hours and days to equilibrate. In respiratory disturbances, therefore, the degree of correction achieved by the metabolic system can tell us something about the duration of the abnormality.
As a general principle, physiological compensatory mechanisms don’t overcompensate; in fact, they often stop just short of total correction. This is a useful fact to remember when trying to interpret a blood gas result that displays both respiratory and metabolic changes. If the pH is in the normal range, it may be difficult to determine which is the primary abnormality and which the compensatory response. Look again at the pH. If the pH is towards the higher end of the normal range, the primary abnormality is probably an alkalosis; if at the lower end then the primary disturbance is an acidosis.
In reading the following examples of acid/base disturbance, refer to the diagrams in Figs 3.9and 3.10.
Respiratory acidosis (acute): pH reduced, PCO2 raised, bicarbonate normal
A reduction in alveolar ventilation causes an increase in arterial PCO 2. The pH falls. In the short term, there is insufficient time for metabolic (renal) correction, so the bicarbonate concentration remains almost unchanged. This pattern is seen where there is a sudden reduction in ventilation, such as obstruction of the airway, overdose of sedative drugs or acute neurological damage.
Respiratory acidosis (chronic): pH normal (lower half of normal range), PCO2 raised, bicarbonate high
If underventilation, from whatever cause, is sustained beyond a few days, renal tubular reabsorption of bicarbonate will achieve a significant elevation in plasma bicarbonate level, which will correct the acidosis caused by the underventilation. This can be caused by any process that results in sustained hypoventilation (commonly seen in COPD).
Читать дальше