High-Performance Materials from Bio-based Feedstocks
Здесь есть возможность читать онлайн «High-Performance Materials from Bio-based Feedstocks» — ознакомительный отрывок электронной книги совершенно бесплатно, а после прочтения отрывка купить полную версию. В некоторых случаях можно слушать аудио, скачать через торрент в формате fb2 и присутствует краткое содержание. Жанр: unrecognised, на английском языке. Описание произведения, (предисловие) а так же отзывы посетителей доступны на портале библиотеки ЛибКат.
- Название:High-Performance Materials from Bio-based Feedstocks
- Автор:
- Жанр:
- Год:неизвестен
- ISBN:нет данных
- Рейтинг книги:5 / 5. Голосов: 1
-
Избранное:Добавить в избранное
- Отзывы:
-
Ваша оценка:
- 100
- 1
- 2
- 3
- 4
- 5
High-Performance Materials from Bio-based Feedstocks: краткое содержание, описание и аннотация
Предлагаем к чтению аннотацию, описание, краткое содержание или предисловие (зависит от того, что написал сам автор книги «High-Performance Materials from Bio-based Feedstocks»). Если вы не нашли необходимую информацию о книге — напишите в комментариях, мы постараемся отыскать её.
High-Performance Materials from Bio-based Feedstocks
The latest advancements in the production, properties, and performance of bio-based feedstock materials
www.wiley.com/go/rrs High-Performance Materials from Bio-based Feedstocks
High-Performance Materials from Bio-based Feedstocks
High-Performance Materials from Bio-based Feedstocks — читать онлайн ознакомительный отрывок
Ниже представлен текст книги, разбитый по страницам. Система сохранения места последней прочитанной страницы, позволяет с удобством читать онлайн бесплатно книгу «High-Performance Materials from Bio-based Feedstocks», без необходимости каждый раз заново искать на чём Вы остановились. Поставьте закладку, и сможете в любой момент перейти на страницу, на которой закончили чтение.
Интервал:
Закладка:
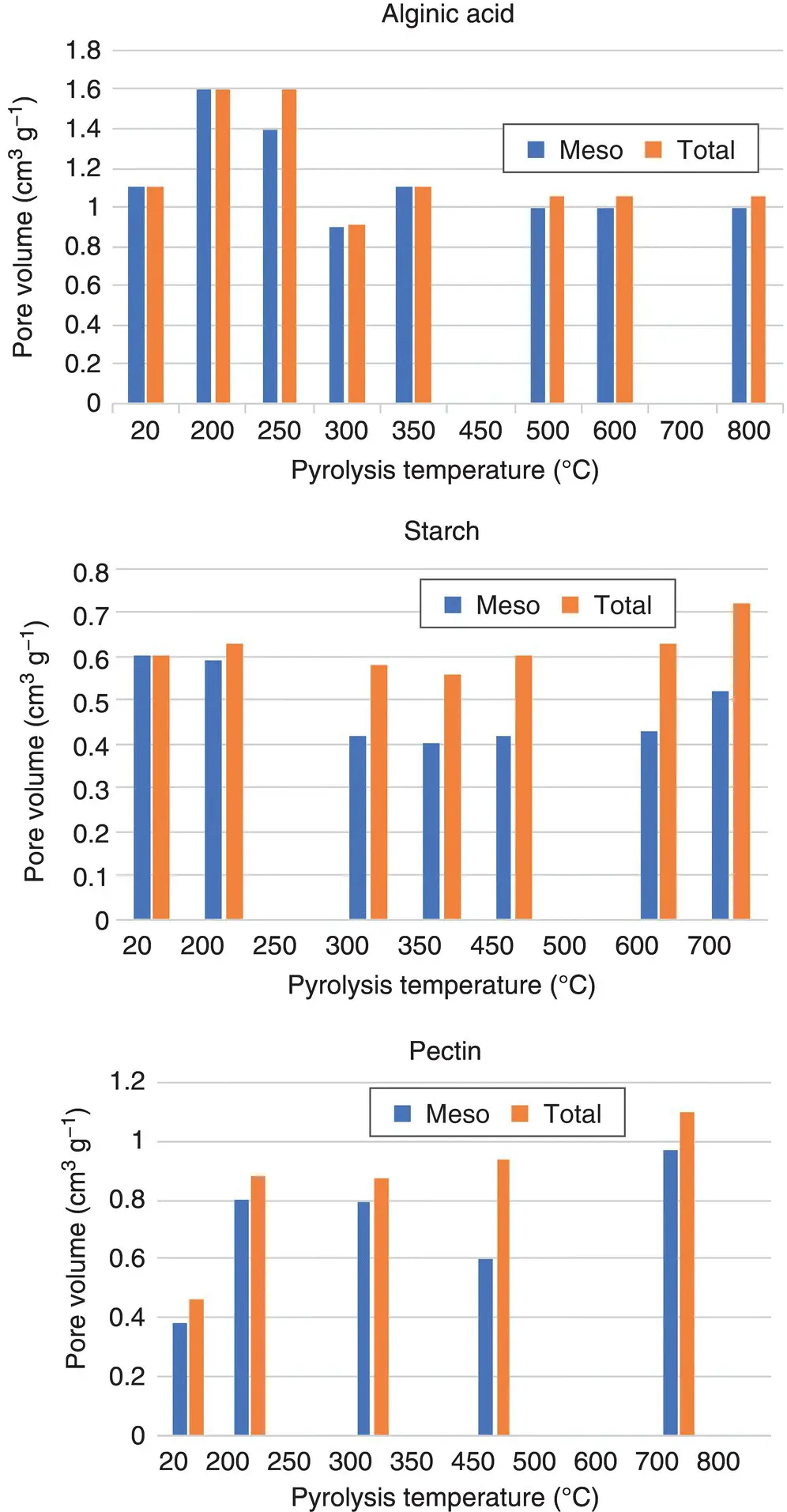
Figure 3.3 Comparison of porosity of the three major Starbon types.
Source: Original data adapted from Budarin et al. [9], White et al. [10], White et al. [11].
A more recent publication expanded on this synthesis by variation of the sulphonating agent and suspension times [20]. In this report, Starbon‐300 was chosen as the base material. The first synthesis was similar to that previously reported, except that a ratio of 0.2 g Starbon to 10 ml H 2SO 4(96%) was used, and a suspension time of 15 hours. Further syntheses were reported using a mixture of ClSO 3H/H 2SO 4as the sulphonating agent, in ratios of 2 : 10 and 3 : 10 by volume, respectively. In these cases, sulphonation was carried out at reflux for five hours.
3.2.2.1.2 Characterisation of Sulphonated Material
Sulphonated Starbon has been analysed thoroughly in terms of porosity, elemental composition, and SO 3H loading: all characteristics affecting the validity of these materials as solid acid catalysts. In the original publication, the SO 3H loading on Starbon‐400 was reported as 0.5 mmol g −1as determined by thermogravimetry coupled to infrared spectroscopy (thermogravimetry‐infra red [TGIR]) [17]. Further investigation showed that the loading remained in this region for preparation temperatures of 600 °C and below. Starbon‐650 and above resulted in a lower concentration of active acid sites (0.3–0.4 mmol g −1) [18, 21]. This was further corroborated by pyridine absorption experiments showing that the acidity of sulphonated Starbon did not change considerably with preparation temperatures of 600 °C or less. However, stronger Lewis acidity was observed at lower temperatures [18, 19]. Later, Aldana‐Pérez et al. reported a –SO 3H content of 1.2, 1.8, and 2.3 mmol g −1, and a total number of acid sites as 8.0, 8.2, and 10 mmol g −1, respectively, for Starbon‐300 sulphonated by H 2SO 4for 15 hours, a mixture of 2 : 10 ClSO 3H/H 2SO 4for five hours, and a mixture of 3 : 10 ClSO 3H/H 2SO 4for five hours [20]. In this case, acidity was measured by potentiometric titration.
Transmission electron microscopy (s) and scanning electron microscopy (SEM) imaging of Starbon have shown that sulphonation did not considerably change the particle size or morphology of the material, which is amorphous after functionalisation, but the structure was prone to cracking at higher carbonisation temperatures as is true for the unsulphonated Starbon [18]. Starbon‐400 was shown to remain predominantly mesoporous (pore size 5–15 nm) after sulphonation, although the mean pore diameter and surface area decreased. As with the original Starbon, microporosity increased with preparation temperatures above 500 °C [19, 21]. A homogeneous distribution of elements was also observed [20]. Additional N 2adsorption studies showed that the average pore diameter of sulphonated Starbon was 8–12 nm [21]. Sulphonated Starbon‐400 had a Brunauer‐Emmett‐Teller (BET) surface area of 386 m 2g −1and pore volume of 0.62 cm 3g −1. Aldana‐Pérez et al. reported a decrease in BET surface area on sulphonation from 163 m 2g −1for Starbon‐300 to 66, 75, and 77 m 2g −1, respectively, for Starbon‐300 sulphonated by H 2SO 4for 15 hours, a mixture of 2 : 10 ClSO 3H/H 2SO 4for five hours, and a mixture of 3 : 10 ClSO 3H/H 2SO 4for five hours [20].
Diffuse reflectance infra red (DRIFT IR) analysis of the original sulphonated Starbon‐400 showed little change in surface structures before and after sulphonation [18, 21]. Peaks in the region of 1300–600 cm −1were attributed to –SO 3H groups confirming the incorporation of sulphur into the Starbon structure, although differentiation between O–SO 3H and C–SO 3H was not possible. Aldana‐Pérez et al. performed fourier Transform infra red (FTIRP) photoacoustic spectra – an infrared technique particularly well suited to study dark samples – of the sulphonated and presulphonation Starbon, as shown in Table 3.1[20]. In particular, this confirmed the presence of –SO 3groups in the sulphonated Starbon by the appearance of the S=O band at approximately 1040 cm −1. Additionally, there is a reduction in the CH 2/CH 3signal at approximately 3000 cm −1, which was attributed to oxidation of aliphatic carbons to carboxylic acids, although oxidation to ketones should also not be excluded in Starbon‐300, where significant carbohydrate character (in particular –CH(OH)– groups) should remain.
Table 3.1 Summarised IR data of a range of sulphonated and unsulphonated Starbon.
Source: From Aldana‐Pérez et al. [20].
| Wavenumber (cm −1) | Vibration | Appearance |
|---|---|---|
| 1040 | S=O symmetric stretch | Sulphonated |
| 1610 | Aromatic C=C stretch | Both |
| 1719 | C=O stretch | Both |
| 3000 | CH 2/CH 3 | Unsulphonated |
| 3440 | C(O) OH /Ph OH stretch | Both |
Solid and liquid 13C NMR data were also collected for the sulphonated catalyst, Starbons‐300‐2–H 2SO 4–ClSO 3H–TEPO, using triethylphosphine oxide (TEPO) as a probe molecule to find the relative acid strength [20]. This was done by dissolving 0.05 g of material in 5 ml of 1 M TEPO in hexane, stirring for one hour, followed by drying at 40 °C under vacuum. The resulting solid was dissolved in deuterated chloroform. Having managed to get better resolution of signals in the liquid state, bands at 128.94, 131.05, and 132.54 ppm were assigned to aromatic carbons while band 167.94 ppm was assigned to carboxylic carbons. The 31P MAS NMR (magic angle spinning nuclear magnetic resonance) of the same material showed the shift of a signal from 53.89 ppm in the unsulphonated Starbon to 85.30 ppm in the sulphonated material, which was attributed to the change from hydroxyl to sulphonic groups, whose interaction with TEPO confirmed strong Brønsted acid character.
Elemental analysis of the original sulphonated Starbon gave a sulphur content of 1.9% (Starbon‐400‐SO 3H), 1.4% (Starbon‐650‐SO 3H), and 1.3% (Starbon‐750‐SO 3H) [21], whereas Aldana‐Pérez et al. reported a higher sulphur content of 3.30% by X‐ray photoelectron spectroscopy (XPS), possibly due to the much longer duration of sulphonation in their method [20].
3.2.2.2 N‐Starbons
3.2.2.2.1 Methods of N Incorporation
Attard et al. first reported a method for the N‐doping of Starbon in 2018 [12] by using chitosan (a natural N‐containing polysaccharide) or ammonia as nitrogen sources. Three methods were described. Route A involved the gelation of chitosan and alginic acid together in the formation of Starbon. Routes B and C involved injecting ammonia into the Starbon at the aerogel stage and into the final product, respectively, where ammonia was adsorbed onto the surface of the material. These N‐doped materials are collectively referred to as N‐Starbon.
Another study used the impregnation of Starbon with monoethanolamine [22]. This involved mixing Starbon derived from both corn and potato starch with a known amount of amine in a solution of ethanol and water. The product was subsequently dried at 105 °C.
3.2.2.2.2 Characterisation
In the account by Attard et al. [12], N‐doped Starbon produced by either the inclusion of chitosan or ammonia displayed similar characteristics. A significant amount of nitrile was observed in these materials, something that was known to be unusual in other forms of N‐doped carbons [12]. The TGIR results showed that chitosan released ammonia upon heating to low temperatures, whereas N‐Starbon did not. It was concluded that the amine groups in chitosan may become trapped in the form of nitriles, the presence of which was confirmed by a DRIFT absorbance band at 2210 cm −1. The nitrile was assumed chemically bonded to the Starbon as it remained present after generous washing with ethanol and water.
Читать дальшеИнтервал:
Закладка:
Похожие книги на «High-Performance Materials from Bio-based Feedstocks»
Представляем Вашему вниманию похожие книги на «High-Performance Materials from Bio-based Feedstocks» списком для выбора. Мы отобрали схожую по названию и смыслу литературу в надежде предоставить читателям больше вариантов отыскать новые, интересные, ещё непрочитанные произведения.
Обсуждение, отзывы о книге «High-Performance Materials from Bio-based Feedstocks» и просто собственные мнения читателей. Оставьте ваши комментарии, напишите, что Вы думаете о произведении, его смысле или главных героях. Укажите что конкретно понравилось, а что нет, и почему Вы так считаете.












