High-Performance Materials from Bio-based Feedstocks
Здесь есть возможность читать онлайн «High-Performance Materials from Bio-based Feedstocks» — ознакомительный отрывок электронной книги совершенно бесплатно, а после прочтения отрывка купить полную версию. В некоторых случаях можно слушать аудио, скачать через торрент в формате fb2 и присутствует краткое содержание. Жанр: unrecognised, на английском языке. Описание произведения, (предисловие) а так же отзывы посетителей доступны на портале библиотеки ЛибКат.
- Название:High-Performance Materials from Bio-based Feedstocks
- Автор:
- Жанр:
- Год:неизвестен
- ISBN:нет данных
- Рейтинг книги:5 / 5. Голосов: 1
-
Избранное:Добавить в избранное
- Отзывы:
-
Ваша оценка:
- 100
- 1
- 2
- 3
- 4
- 5
High-Performance Materials from Bio-based Feedstocks: краткое содержание, описание и аннотация
Предлагаем к чтению аннотацию, описание, краткое содержание или предисловие (зависит от того, что написал сам автор книги «High-Performance Materials from Bio-based Feedstocks»). Если вы не нашли необходимую информацию о книге — напишите в комментариях, мы постараемся отыскать её.
High-Performance Materials from Bio-based Feedstocks
The latest advancements in the production, properties, and performance of bio-based feedstock materials
www.wiley.com/go/rrs High-Performance Materials from Bio-based Feedstocks
High-Performance Materials from Bio-based Feedstocks
High-Performance Materials from Bio-based Feedstocks — читать онлайн ознакомительный отрывок
Ниже представлен текст книги, разбитый по страницам. Система сохранения места последней прочитанной страницы, позволяет с удобством читать онлайн бесплатно книгу «High-Performance Materials from Bio-based Feedstocks», без необходимости каждый раз заново искать на чём Вы остановились. Поставьте закладку, и сможете в любой момент перейти на страницу, на которой закончили чтение.
Интервал:
Закладка:
The first methodology utilised solvent exchange – water has a much higher surface tension (72.74 mN m −1at 20 °C [13]) than most organic liquids (e.g. acetone 23.70 mN m −1, ethanol 22.27 mN m −1, and methanol 22.60 mN m −1[14]).
Therefore, exchanging water first for ethanol by simple mixing and filtration (×3) led to approximately 12% water in ethanol‐filled gel, which had to be further exchanged with acetone using the same methodology in order to provide an aerogel with porosity intact. What is interesting here is that the gel produced after the ethanol exchanges contains a liquid (c. 12% water in ethanol) which has a surface tension (22.6 mN m −1) [15] very close to that of acetone (23.70 mN m −1) [14], yet fails to lead to a satisfactory material. While this may be due to the evaporation of an ethanol‐rich vapour (meaning that the remaining liquid is enriched in water, leading to the surface tension of the remaining liquid increasing and causing damage), more extensive ethanol washing does not lead to better materials. Similarly, direct exchange with acetone does not provide acceptable materials. Thus, both solvents must be used for a successful material.
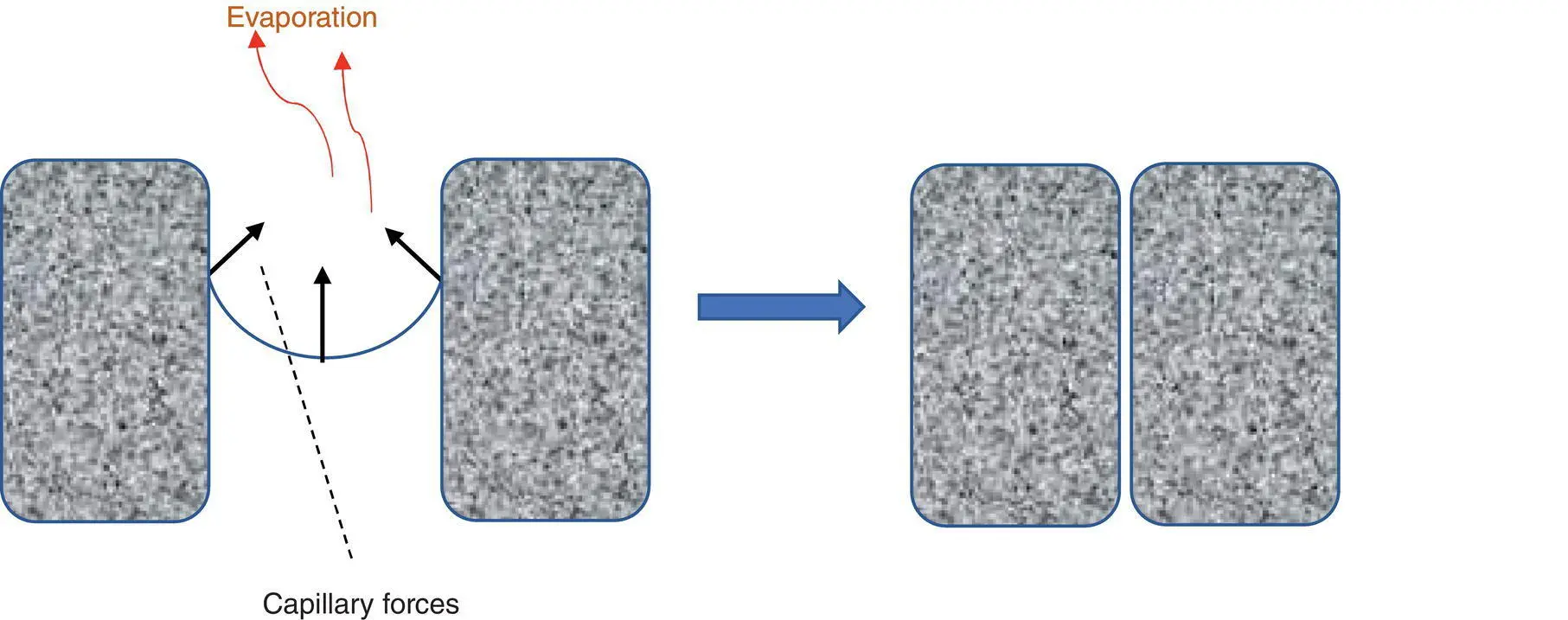
Figure 3.1 Role of capillary forces in the collapse of soft porous materials.
A major downside to this approach (apart from cost) is the large volume of mixed solvents that are very difficult to purify and reuse. While the materials obtained are very good, the process lacks environmental credentials.
In order to develop a more efficient, less solvent‐intensive process, Borisova et al. developed a route involving freeze‐drying of hydrogels doped with t‐butanol, a molecule often utilised in freeze‐drying to control the freezing process [16]. This approach avoids surface tension issues as the phase transition is from solid to gas, and the capillary forces that plague direct drying are thus avoided.
The optimal porosity characteristics were achieved at the eutectic points of the water – t‐BuOH system, with a more distinct optimum found at the water‐rich eutectic (c. 23% t‐butanol). At this solvent composition, the macroporosity that dominates in freeze‐drying of the pure water hydrogels is strongly reduced and considerable mesoporosity is developed. While all three polysaccharides display qualitatively similar behaviour, the values of mesopore volume (pectin: 3.1 cm 3g −1; alginic acid: 1.8 cm 3g −1; starch: 1.1 cm 3g −1) are quite different, but nonetheless impressive, in each case ( Figure 3.2).
Therefore, compared with a solvent exchange, the quality of the materials (in terms of mesopore content) is excellent, and much less solvent is required as the t‐butanol can be recovered and reused.
3.2.1.3 Pyrolysis of the Expanded Aerogel
The final stage of pyrolysis involves a slow temperature ramp from room temperature to the desired temperature. This drives off water, a complex bio‐oil (typically formed between 250 and 400 °C) and smaller gases (CO 2, CO, etc.) which are the dominant species at higher temperatures. With increasing temperature, the nature of the surface changes from polysaccharide‐like, gradually increasing the C : O ratio from 1 : 1 to around 30 : 1 at 800 °C. The increasingly carbon‐like nature of the material underlies profound changes in chemistry. While these changes are complex and not fully understood, globally it can be said that, for starch at least, initial dehydrations occur which lead to unsaturation (C=C and C=O functionality) leading to an enhanced reactivity. These groups react further, cross‐linking and releasing small organic molecules into the gas phase to form a broadly aromatic matrix [9]. This is somewhat of an oversimplification as significant aliphatic functionality is always seen by 13C NMR. This may be due, in part at least, to the early dehydration of sugars, which can lead initially to –CH 2–C(=O)– units, although the reactivity of such groups suggests that more structural features of greater stability are initially formed. After the initial substantial mass loss, further heating causes much more gradual mass loss, predominantly in the form of small molecules such as water, CO, and CO 2.
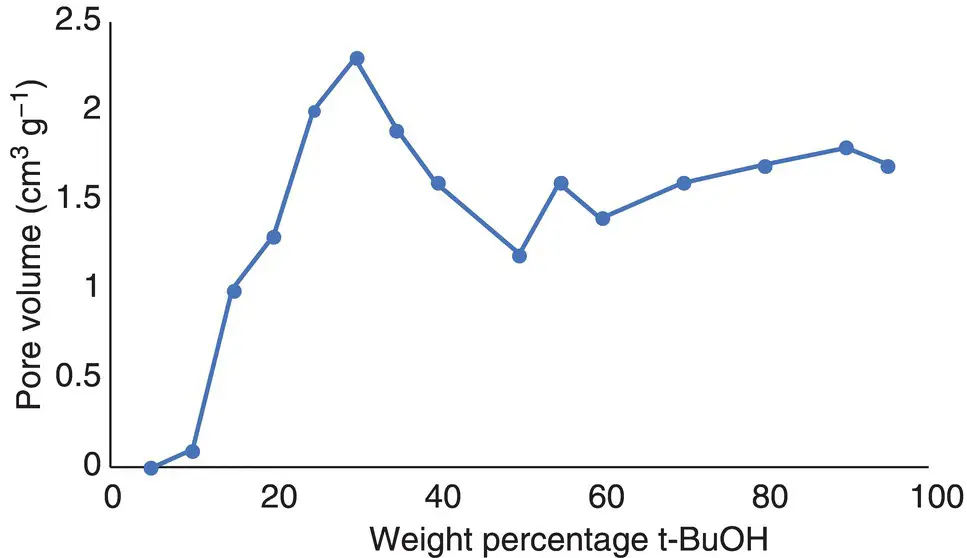
Figure 3.2 Evolution of porosity as a function of water : butanol composition. Eutectic points are at 25 wt% t‐BuOH and at 95 wt% t‐BuOH.
Source: Original data from Borisova et al. [16].
Thus, the chemical functionality of the surface of the Starbon materials represents a continuum of changing functionality ranging from hydroxylic to highly functional (hydroxylic, unsaturated) to a more aromatic, low oxygen structure.
The mesoporosity of the materials compared to the total (meso + micro) porosity are shown in Figure 3.3for the three materials derived from alginic acid, starch, and pectin [9–11].
What can be seen from a comparison of the three types of materials is that, overall, the pore volumes remain fairly constant over a wide temperature range. However, in the case of alginic‐acid‐derived materials, there is an increase at lower temperatures followed by a drop and then relative constancy. For room temperature pectin, there is evidence of increasing porosity. The total volumes are broadly constant over all three material types. The most variation is in the difference between total and mesopore volumes, indicating the extent of microporosity. Alginic‐acid‐derived materials have virtually no microporosity at any temperature and pectin a modest amount. In contrast, starch‐derived materials display very little microporosity at low pyrolysis temperatures, but from 300 °C onwards, the materials develop a considerable amount (up to c. 30%).
Critical to maintaining the porosity of the materials is that the pyrolysis and cross‐linking reactions occur before the aerogel melts or softens considerably. For alginic acid and pectin, the more reactive nature of the polysaccharides’ structure – acid and ester groups – means that this is not an issue, while for starch, a strong Brønsted acid (typically p‐toluene sulphonic acid) must be added.
3.2.2 Derivatisation
Since the development of Starbon as a high‐surface‐area mesoporous carbon, multiple reports have been published on its use as solid catalyst support. Starbon itself has a very modest catalytic activity – as will be seen next, there is little more than some weakly acidic groups on the surface. Therefore, the addition of more powerfully active groups (e.g. sulphonic acid sites or basic amines) is likely to provide a much more active material for catalytic applications, while N functionalised materials may also improve adsorbency of metals and function as ligands for catalytically active surfaces.
3.2.2.1 Sulphonation
3.2.2.1.1 Method of Sulphonation
The first published method of Starbon sulphonation was described by Budarin et al. [17]. In this synthesis, sulphonation was carried out on Starbon having been pre‐carbonised at 400 °C (Starbon‐400) as this was considered to have the best ratio of hydrophilic and hydrophobic functional groups. The Starbon‐400 was heated for four hours at 80 °C in a suspension of H 2SO 4(99.999% purity) at a ratio of 10 ml acid to 1 g Starbon, and the sulphonated material was subsequently washed with distilled water until no further acid was leached. This was followed by conditioning in toluene for four hours at 150 °C and then in water for three hours at 100 °C. The resultant solid acid was dried overnight in an oven at 100 °C. Subsequent publications from the same authors noted a reduction in the aqueous conditioning temperature to 80 °C [18, 19].
Читать дальшеИнтервал:
Закладка:
Похожие книги на «High-Performance Materials from Bio-based Feedstocks»
Представляем Вашему вниманию похожие книги на «High-Performance Materials from Bio-based Feedstocks» списком для выбора. Мы отобрали схожую по названию и смыслу литературу в надежде предоставить читателям больше вариантов отыскать новые, интересные, ещё непрочитанные произведения.
Обсуждение, отзывы о книге «High-Performance Materials from Bio-based Feedstocks» и просто собственные мнения читателей. Оставьте ваши комментарии, напишите, что Вы думаете о произведении, его смысле или главных героях. Укажите что конкретно понравилось, а что нет, и почему Вы так считаете.












