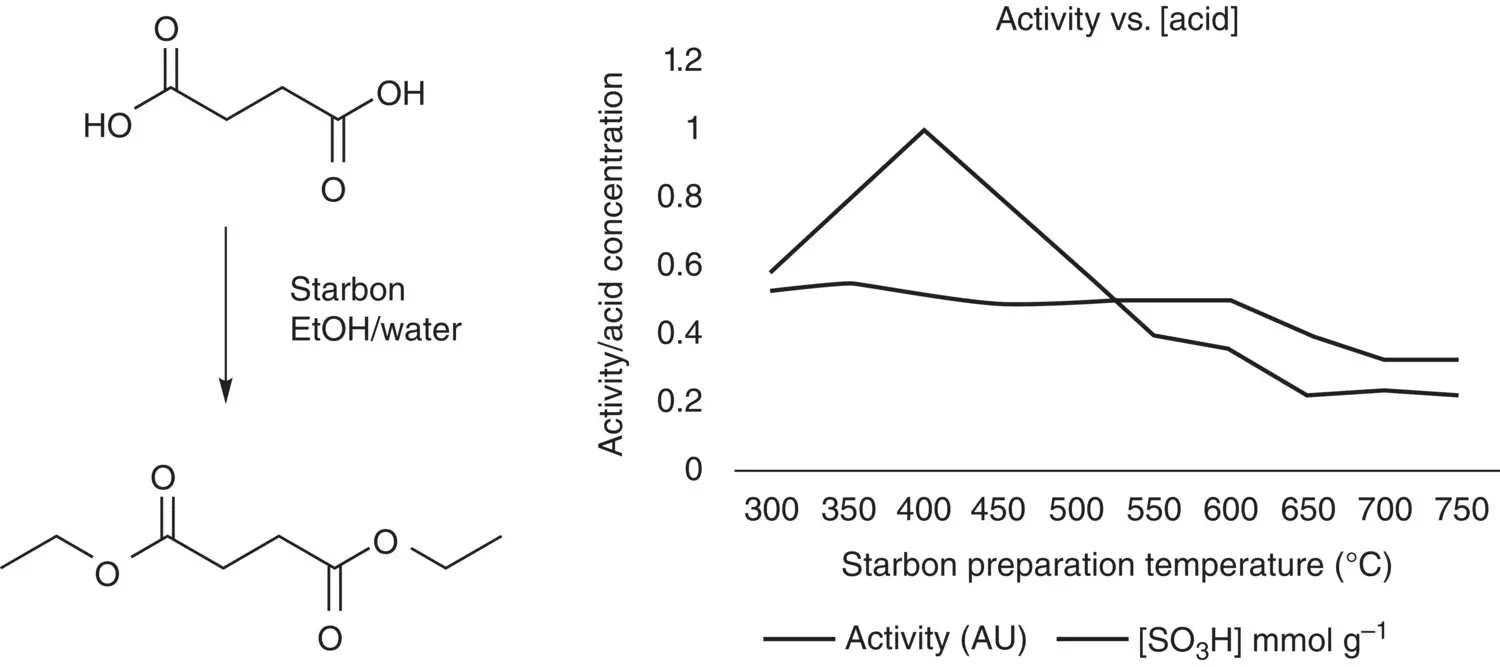
Figure 3.4 Diesterification of succinic acid in aqueous ethanol as a function of Starbon preparation temperature.
Source: Data from Clark et al. [19].
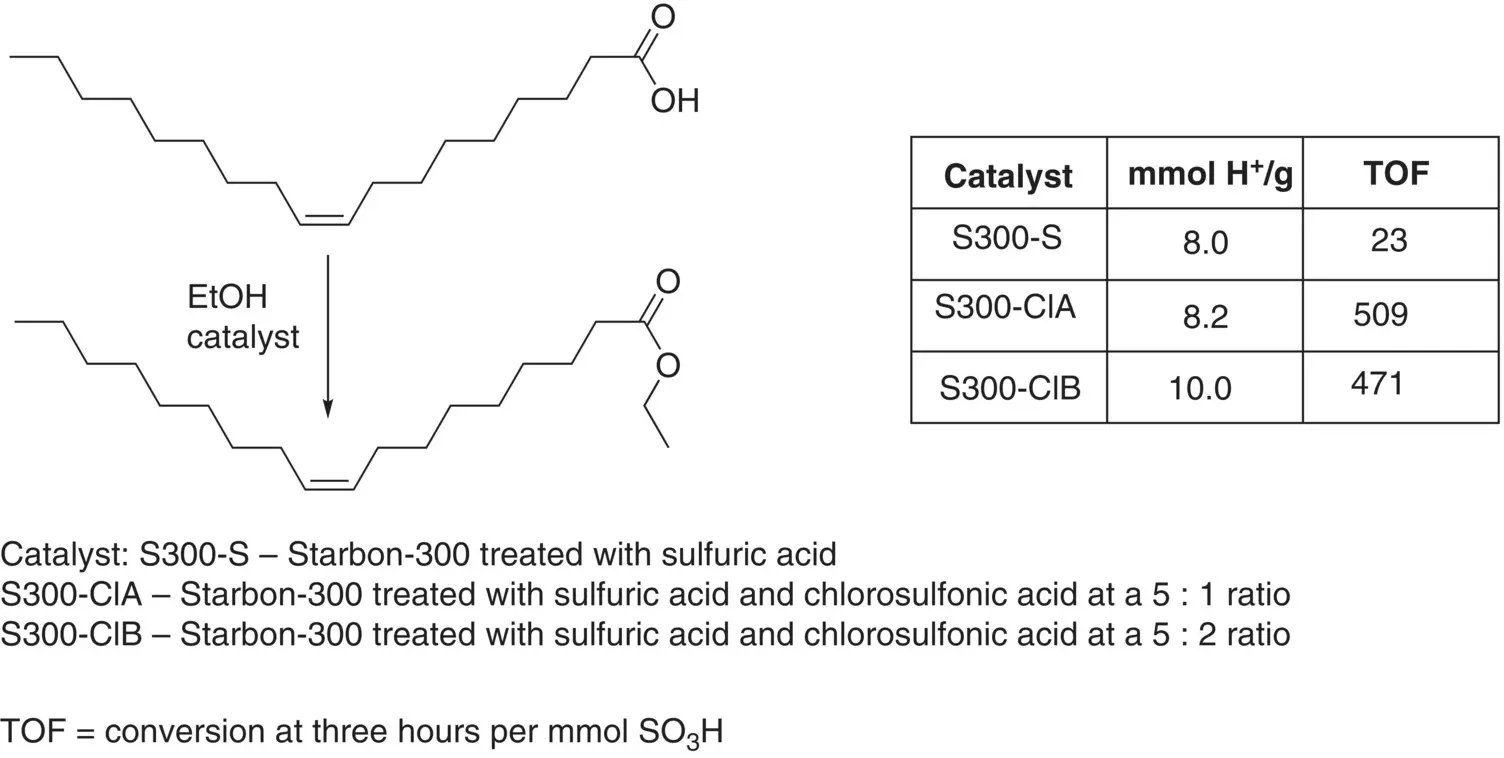
Figure 3.5 Esterification of oleic acid with sulphonated Starbon materials.
Source: Data from Aldana‐Pérez et al. [20].
3.2.3.1.2 Sulphonated Starbon in Dehydrations
Millán et al. [26] have demonstrated the use of Starbon‐450‐SO 3H in the dehydration of xylose to furfural in biphasic conditions. They found that, in a single aqueous phase, conversion of xylose was readily achieved, but furfural was further degraded, meaning selectivity and yield of furfural was low (38% yield and 65% selectivity at best). The addition of cyclopentyl methyl ether (CPME) as a water‐immiscible co‐solvent was found to aid the reaction considerably by rapidly removing furfural from the aqueous solution, leading to optimal performance of 96% conversion and 72.5% selectivity after one hour at 200 °C ( Figure 3.6). Interestingly, given the rather challenging conditions, reusability (measured at 175 °C for 18 hours) was excellent, with no change in performance over 3 runs.
3.2.3.1.3 Sulphonated Starbon in Amide Synthesis
Starbon‐400‐SO 3H has been used to form amides in excellent yields from a range of anilines and acetic acid under microwave irradiation [27].
After 15 minutes, at a maximum temperature of 130 °C, yields approaching quantitative were achieved for a range of aliphatic amines and anilines. Interestingly, the simplest primary amines (C 5, C 6, and C 10) tended to give slightly poorer yields, despite them being typically more active than aromatic systems. As expected in amide formation, selectivity was excellent. Other aliphatic acids were also screened with very good results, with secondary acids being slightly less reactive on steric grounds. Other strong solid acids were investigated with significantly lower conversions.
The same catalyst was utilised by Mesquita et al. in the acid‐catalysed Ritter reaction of steroids, again forming amide products [28]. Starting from epoxy steroids and acetonitrile, the authors found that Starbon‐400‐SO 3H catalysed the ring opening, and trapping of the resultant carbocation via hydration gave the resultant product as shown in Figure 3.7.
A series of different steroids was successfully functionalised in yields of 88–96%, with a lower yield of 60% being obtained with a steroidal epoxide of the opposite configuration to that shown in Figure 3.7. Similar yields were obtained when methylthioacetonitrile (MeSCH 2CN) was used, with the product being the MeSCH 2C(O)N‐product. With MeSO 2CH 2CN as the amide, oxazoline was formed by trapping of the intermediate carbocation (formed by attack of the nitrile on the protonated epoxide) by the vicinal hydroxy group.
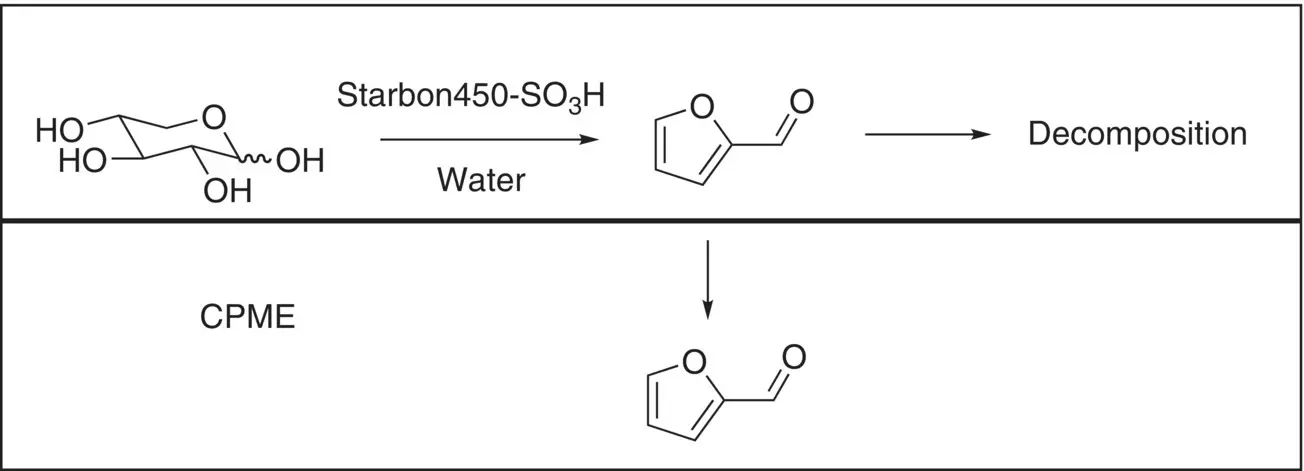
Figure 3.6 Conversion of xylose to furfural and extraction of furfural.
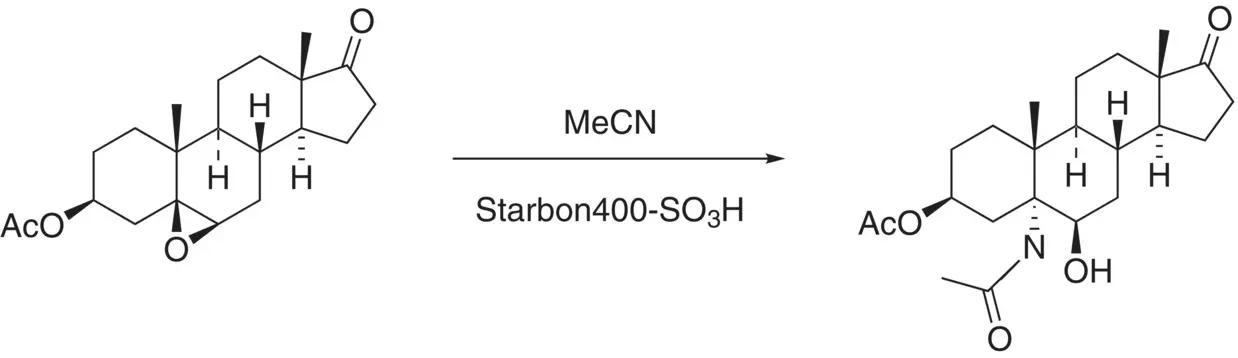
Figure 3.7 Functionalisation of steroids via Starbon acid‐catalysed Ritter reaction.
3.2.3.1.4 Sulphonated Starbon in Acylations and Alkylations
Luque et al. have also published research on a range of different Starbon acids, with sulphonic and carboxylic groups attached, as well as ZnCl 2and BF 3adsorbed onto the surface [29]. They characterised the nature of the acidity by pyridine titration and found that the sulphonic acid systems had both Brønsted and Lewis acidity. In contrast, the two Lewis acid‐doped materials had a greater Lewis acidity (as expected) but an unexpectedly low quantity of acid sites. The various materials were tested in the acetylation of 5‐acetyl methyl salicylate, and were found to be very active in the O‐acetylation, with Friedel Crafts acylation being absent despite the presence of Lewis acidic sites ( Figure 3.8a). Alkylation of phenol with cyclohexene was also investigated ( Figure 3.8b). While O‐alkylation was very dominant, especially after short reaction times, C alkylation (mainly in the ortho position) increased upon prolonged reaction. The reasons for this are not discussed in the paper, but similar behaviour is seen with some other catalysts. This may be due to a change in the nature of the catalyst with time, or more likely, to a slow Fries rearrangement reaction following a rapid O‐alkylation.
Further alkylations (of toluene and xylenes) with benzyl chloride have also been successfully carried out using Starbon‐400‐SO 3H under microwave conditions. High yields (70–95%) were achieved after 15 minutes for toluene and p‐xylene, while longer times were required for m‐xylene and, surprisingly, anisole [17].
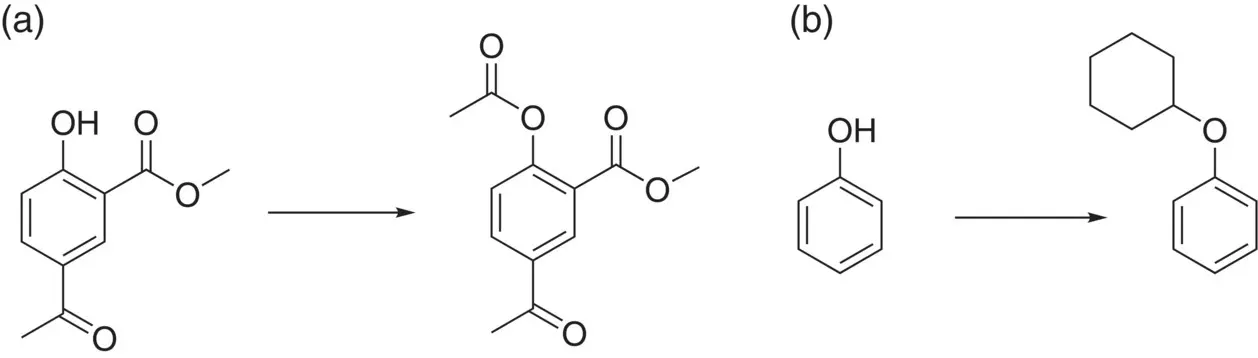
Figure 3.8 Friedel Crafts reactions catalysed by a range of Starbon acids. (a) O‐Acylation of a phenol and (b) O‐alkylation of a phenol.
3.2.3.1.5 Supported Metal Complexes
Only a very few examples of supported metal complexes and their catalytic activity have been reported, perhaps surprising, given the large numbers that exist with silica as support. Interestingly, little has been done to extend the well‐established organo‐silica chemistry beyond the initial studies by Doi et al. [30] who used expanded starch (i.e. non‐pyrolysed Starbon) as a support. Two approaches that successfully attach complex catalytic species to the surface of Starbon are available, and are discussed next.
Matharu et al. [23] published details of an Fe‐NHC catalytic system (NHC = N‐heterocyclic carbene), which they tethered to the surface of a starch‐based Starbon‐350 and also to the surface of the precursor‐expanded starch ( Figure 3.9). They utilised a multistage ligand synthesis, anchored the ligand to the surface, and then finally attached the metal species [23].
The synthesis of the heterocyclic ligand containing amine functionality as an anchor was carried out. Separately, Starbon surface was functionalised with a succinimyl carbonate group, following an adapted literature procedure, where the toxic dimethyl formamide (DMF) solvent was replaced with propylene carbonate, a safer alternative to dipolar aprotics [31]. Finally, the ligand was bound to the functionalised Starbon surface. Anchoring of the ligand system was achieved by reaction of the functionalised Starbon with the amine pendant on the ligand moiety. The degree of substitution achieved for the succinimidyl carbonate grafting was 0.33, approximating to 1 in 10 hydroxyls being substituted (in the case of the expanded starch, this is likely to be somewhat higher for the Starbon‐350, although the complexity of the structure is much greater with a wider range of functionalities). Given the extensive H‐bonding and steric hindrance pertinent to the majority of the hydroxyls in such polysaccharides, this is a reasonably significant degree of substitution, and led to metal centre loadings of 0.26 and 0.3 mmol g −1, well within the range of loadings achieved for highly porous silicas.
Читать дальше

















