Many examples of silicate crystal structures are given in the minerals section of the CrystalViewer Companion website. To facilitate viewing of the silicate anion, the remaining cations in the structures can be hidden; you can check out the numbers of bridging and non‐bridging oxygens for consistency with the Si–O ratio in the mineral formulae.
The structural building blocks of clay minerals are illustrated in Fig. 1.52(d, e, f). They consist of layers of silicate or aluminosilicate tetrahedra which all have the same orientation; their apices are connected to layers of Al, Mg octahedra to form either (d) double- or (e,f) triple-layered sheets. Clay minerals form in nature by the decomposition of feldspars such as microcline or orthoclase to give kaolinite:
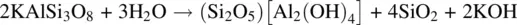
Kaolinite has a two layer, 1:1, structure (d) whose opposite surfaces are the basal planes of Si 2O 5 2−layers and Al 2(OH) 4 2+layers. These double layers are stacked together and separated by weak van der Waals bonds, that give rise to the softness and easy cleavage of such clay minerals. Other layered minerals such as muscovite (e), montmorillonite (f), talc, bentonite and pyrophyllite have a three layer, 2:1, structure in which the layer of octahedra is sandwiched between two layers of tetrahedra. If the overall structure is electrically neutral, then the interlayer, van der Waals space may contain only water molecules (f), but if the layers have a net negative charge then cations such as K +also occupy the interlayer space, as in micas (e).
Kaolinite clays have long‐standing industrial uses as the fundamental components of whitewares and traditional ceramics. The interlayer bonding forces control the rheological properties and viscosity of slips during processing and formation of the green bodies. In a kaolin particle, the opposite faces of the sheets have different chemical properties and respond differently in an aqueous environment. The bases of the silicate tetrahedra are weakly acidic whereas those of the hydroxide octahedra are weakly basic. Further, the effective surface charges change sign at a different pH for the two particle surfaces. Thus, the silicate surface has an isoelectric point, IEP at pH ~ 3 at which the surface charge, or zeta potential, changes sign, whereas the IEP for the hydroxide surface is at pH ~ 10. This means that at neutral pH, for instance, the two surfaces are oppositely charged. Control of surface charges is vital to the dispersion/agglomeration of particles during slip casting and therefore, for the quality of the ceramic product.
There is increasing interest in the synthesis of new functional materials by starting with layered structures such as clay minerals and modifying the components – ionic or molecular – that occupy the interstitial space between the layers. A wide range of materials and properties can be synthesised by chimie douce methods, such as pillared clays that have novel porous structures and magnetic hybrid materials. Such materials are kinetically stable, although thermodynamically metastable and cannot be prepared by the usual high temperature solid state reaction methods (Section 4.3.7.2).
1.18 Point Groups and Space Groups
A working knowledge of point groups and space groups is invaluable for anyone interested in crystal chemistry and structure‐property relations. Without such knowledge, one is entirely dependent for information about crystal structures on the availability of 3D models, crystal structure libraries, or good quality drawings in the literature. Once the basic principles of space groups are grasped, however, it is possible to make drawings of a structure for oneself, from different orientations if required, or even to construct one's own 3D models. All that is needed is a listing of the atomic coordinates in the structure and details of the relevant space group. There are also various excellent commercial software packages, such as CrystalMaker, available which take away much of the hard work involved in preparing structural drawings.
This section is written for the non‐crystallographer; short‐cuts are made and many of the complications and subtleties of space groups are avoided. The main objective is to show the relation between space groups and sets of atomic coordinates on the one hand and 3D crystal structures on the other. A familiarity with unit cells, crystal systems, Bravais lattices and the elements of point and space symmetry, summarised in Sections 1.1– 1.6, is essential background knowledge. We begin with a discussion of point groups . Point groups are much simpler than space groups because the elements of translational symmetry are absent from point groups. They therefore provide a relatively painless introduction to the subject while introducing the necessary concepts at an early stage. We then look at a few selected examples of space groups and finish with two examples that show the derivation of crystal structures from space group and atomic coordinates information.
The elements of point symmetry which may be observed in crystals are the rotation axes 1, 2, 3, 4 and 6, the inversion axes 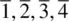 and
and 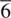 , and the mirror plane, m (which is equivalent to
, and the mirror plane, m (which is equivalent to 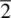 ). These symmetry elements may occur either alone or in various possible combinations with each other to give a total of 32 possible crystallographic point groups . The method of drawing and labelling point groups used here is the same as that recommended in International Tables for X‐ray Crystallography, Vol 1. The symbols for the different point symmetry elements are given in Table 1.28. The thirty‐two point groups, classified according to their crystal system, are listed in Table 1.29 and shown diagrammatically in Appendix E.
). These symmetry elements may occur either alone or in various possible combinations with each other to give a total of 32 possible crystallographic point groups . The method of drawing and labelling point groups used here is the same as that recommended in International Tables for X‐ray Crystallography, Vol 1. The symbols for the different point symmetry elements are given in Table 1.28. The thirty‐two point groups, classified according to their crystal system, are listed in Table 1.29 and shown diagrammatically in Appendix E.
Table 1.28 Point symmetry elements
| Symmetry element |
Written symbol |
Graphical symbol |
| Rotation axes |
1 |
None |
| 2 |
 |
| 3 |
 |
| 4 |
 |
| 6 |
 |
| Inversion axes |
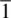 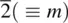 |
None a _____ b |
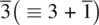 |
 |
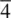 |
 |
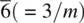 |
 |
| Mirror plane |
m |
____ |
a The inversion axis, 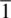 , equivalent to a centre of symmetry, is represented as a small open circle, o, in space groups, but does not have a formal graphical representation in point groups, even though it is present in many point groups.
, equivalent to a centre of symmetry, is represented as a small open circle, o, in space groups, but does not have a formal graphical representation in point groups, even though it is present in many point groups.
Читать дальше

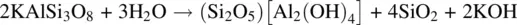
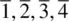 and
and 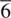 , and the mirror plane, m (which is equivalent to
, and the mirror plane, m (which is equivalent to 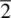 ). These symmetry elements may occur either alone or in various possible combinations with each other to give a total of 32 possible crystallographic point groups . The method of drawing and labelling point groups used here is the same as that recommended in International Tables for X‐ray Crystallography, Vol 1. The symbols for the different point symmetry elements are given in Table 1.28. The thirty‐two point groups, classified according to their crystal system, are listed in Table 1.29 and shown diagrammatically in Appendix E.
). These symmetry elements may occur either alone or in various possible combinations with each other to give a total of 32 possible crystallographic point groups . The method of drawing and labelling point groups used here is the same as that recommended in International Tables for X‐ray Crystallography, Vol 1. The symbols for the different point symmetry elements are given in Table 1.28. The thirty‐two point groups, classified according to their crystal system, are listed in Table 1.29 and shown diagrammatically in Appendix E.



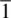
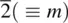
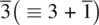

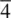

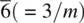

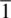 , equivalent to a centre of symmetry, is represented as a small open circle, o, in space groups, but does not have a formal graphical representation in point groups, even though it is present in many point groups.
, equivalent to a centre of symmetry, is represented as a small open circle, o, in space groups, but does not have a formal graphical representation in point groups, even though it is present in many point groups.










