The unit cell of all three structures is hexagonal and has six cp oxygen layers parallel to the basal plane, shown in Fig. 1.46(a) at c heights 1/12, 3/12, 5/12, 7/12, 9/12 and 11/12. Cations are in octahedral sites mid‐way between the oxygen layers; alternate layers of cation sites are occupied by Fe and Ti in ilmenite, Fig. 1.46(b). Pairs of octahedra share a common face in the c direction and cation repulsion between the cation pairs causes distortion from an ideal hcp structure. In all cases, the cation octahedra are distorted with three long and three short bonds. Repulsion between Nb 5+and Li +in LiNbO 3causes displacement of Li to a position near the triangular face at the opposite side of the octahedron. LiNbO 3and LiTaO 3are ferroelectric materials and cation displacements within the face‐sharing octahedra are responsible for the polar crystal structures and dipole reorientation in an applied electric field, which is a characteristic feature of ferroelectrics.
The cation ordering sequence in LiNbO 3is different to that in ilmenite, Fig. 1.46(c) and (d). Li and Nb are both present, ordered, between any pair of close packed oxide ion layers whereas Fe and Ti occupy alternate sets of layers in ilmenite. An alternative view of the LiNbO 3structure is given in (d), which illustrates that LiNbO 3can also be regarded as a grossly distorted perovskite structure. Tilting and rotation of the NbO 6octahedra (B sites) reduce the coordination of the A sites from 12 to distorted octahedral, and these are occupied by Li. If we regard LiNbO 3as a distorted perovskite, its tolerance factor is 0.78, which, in practice, represents the lower limit for materials that can be regarded as distorted perovskites.
1.17.12 Fluorite‐related structures, pyrochlore, weberite and rare earth sesquioxides
The fluorite structure of CaF 2can be described as eutactic ccp Ca 2+ions with F –ions occupying all tetrahedral sites, Fig. 1.29. A number of more complex fluorite‐related structures occur with an excess or deficiency of anions or with cation ordering.
A remarkable anion‐excess fluorite is LaH 3; LaH 2forms the basic fluorite structure and extra hydrogens occupy fully the octahedral sites. The structure therefore has all tetrahedral and octahedral sites occupied by H within a ccp La array, Fig. 1.24. A similar structure is observed in intermetallics such as Li 3Bi and Fe 3Al. These structures represent an extreme with full occupancy of all tetrahedral and octahedral sites. Partial tetrahedral site occupancies occur in the lanthanum hydrides which form a continuous solid solution between LaH 1.9and LaH 3.
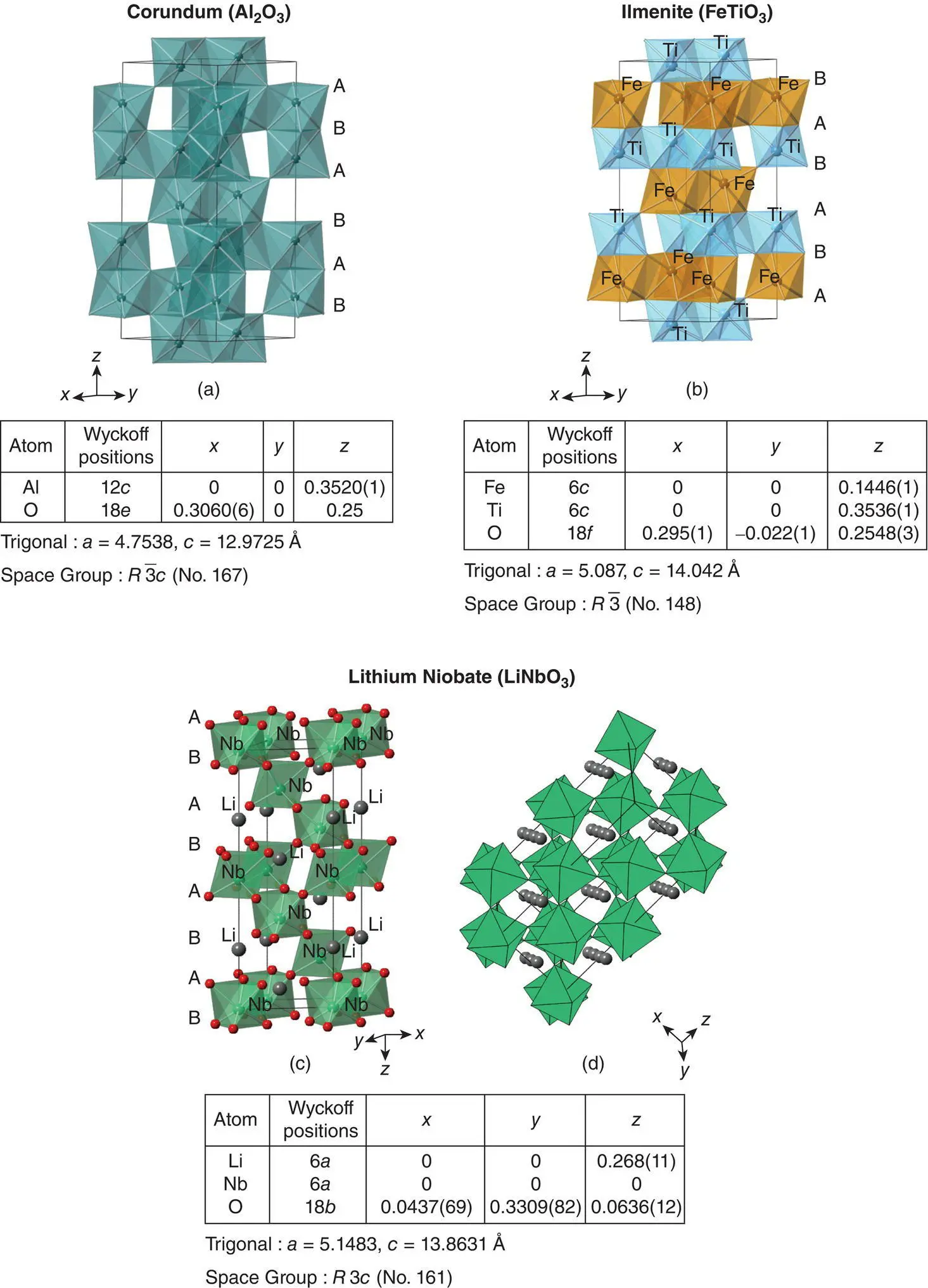
Figure 1.46 Crystal structures of (a) corundum, (b) ilmenite and (c, d) LiNbO3.
Table 1.24 Some compounds with corundum‐related structures
| Corundum |
M 2O 3: M = Al, Cr, Fe (hematite), Ti, V, Ga, Rh |
| ( α ‐alumina) |
Al 2O 3: with Cr dopant (ruby) Al 2O 3: with Ti dopant (sapphire) |
| Ilmenite |
MTiO 3: M = Mg, Mn, Fe, Co, Ni, Zn, Cd MgSnO 3, CdSnO 3NiMnO 3NaSbO 3 |
| LiNbO 3, LiTaO 3 |
|
Oxygen‐excess fluorites occur in UO 2+x(see Fig. 2.10); the structure is distorted locally and the extra oxide ions are displaced off cube body centres. This has a knock‐on effect in which some of the corner oxide ions are displaced onto interstitial sites. The UO 2+xsystem has been studied in considerable detail because of its importance in the nuclear industry as a fuel in nuclear reactors.
Mixed anion oxyfluorides such as LaOF and SmOF form the fluorite structure in which similarly sized O 2–and F –ions are disordered over the tetrahedral sites. Various examples of mixed‐cation fluorites are known in which two different cations are ordered, as shown for several examples in Fig. 1.47. These structures are rather idealised, however, since the anions are displaced off the centres of the tetrahedral sites in various ways to give, for instance, a distorted tetrahedral environment for Cr 2+in SrCrF 4and distorted octahedral coordination for both Ti and Te in TiTe 3O 8.
Li 2O has the antifluorite structure and various Li‐deficient antifluorites are good Li +ion conductors; for instance, Li 9N 2Cl 3has 10% of the Li +sites vacant, giving rise to high Li +ion mobility.
The pyrochlore structure may be regarded as a distorted, anion‐deficient fluorite with two different‐sized cations A and B. Its formula is written as either A 2B 2X 7or A 2B 2X 6X′. The unit cell is cubic with a ~11 Å and contains eight formula units. In principle, the structure is simple since, ideally, it is a fluorite with one‐eighth of the tetrahedral anion sites empty. Also, there is only one variable positional parameter, the x fractional coordinate of the 48 X atoms in the position ( x , 1/8, 1/8), etc., Fig. 1.48. Various compounds form the pyrochlore structure and their differences depend on the value of x , which is usually in the range 0.31–0.36. In the extreme case that x = 0.375, the structure is derived from an undistorted fluorite with 8‐coordinate A cations, as in fluorite, but grossly distorted BX 6octahedra. As x decreases, the 48 X atoms move off their regular tetrahedral sites and the cubic coordination of A becomes distorted: six X neighbours form a puckered hexagon and two X′ atoms are at a different distance, in apical positions, on either side of the puckered hexagon.
At x = 0.3125, the B coordination becomes undistorted octahedral and the BX 6octahedra link by sharing corners to form a 3D network. The A coordination may be described as 2X′ + 6X, with short A–X′ bonds. These A–X′ bonds form a 3D network, A 2X′, that interpenetrates the network of BX 6octahedra, of stoichiometry B 2X 6. The A 2X′ net, with linear X′–A–X units and X′A 4tetrahedra, is similar to that in cuprite, Cu 2O. The B 2X 6and A 2X′ networks, that together form the pyrochlore structure, are shown separately in Fig. 1.48.
Pyrochlore‐based oxides have a range of interesting properties and applications. La 2Zr 2O 7is an electronic insulator whereas Bi 2Ru 2O 7‐δis metallic and Cd 2Re 2O 7is a low temperature superconductor. (Gd 1.9Ca 0.1) Ti 2O 6.9is an oxide ion conductor and Y 2Mo 2O 7is a spin glass. As indicated in some of the above examples, the anion content is not always 7 and indeed the amount of X′ in the general formula can have values between 0 and 1 in different pyrochlores.
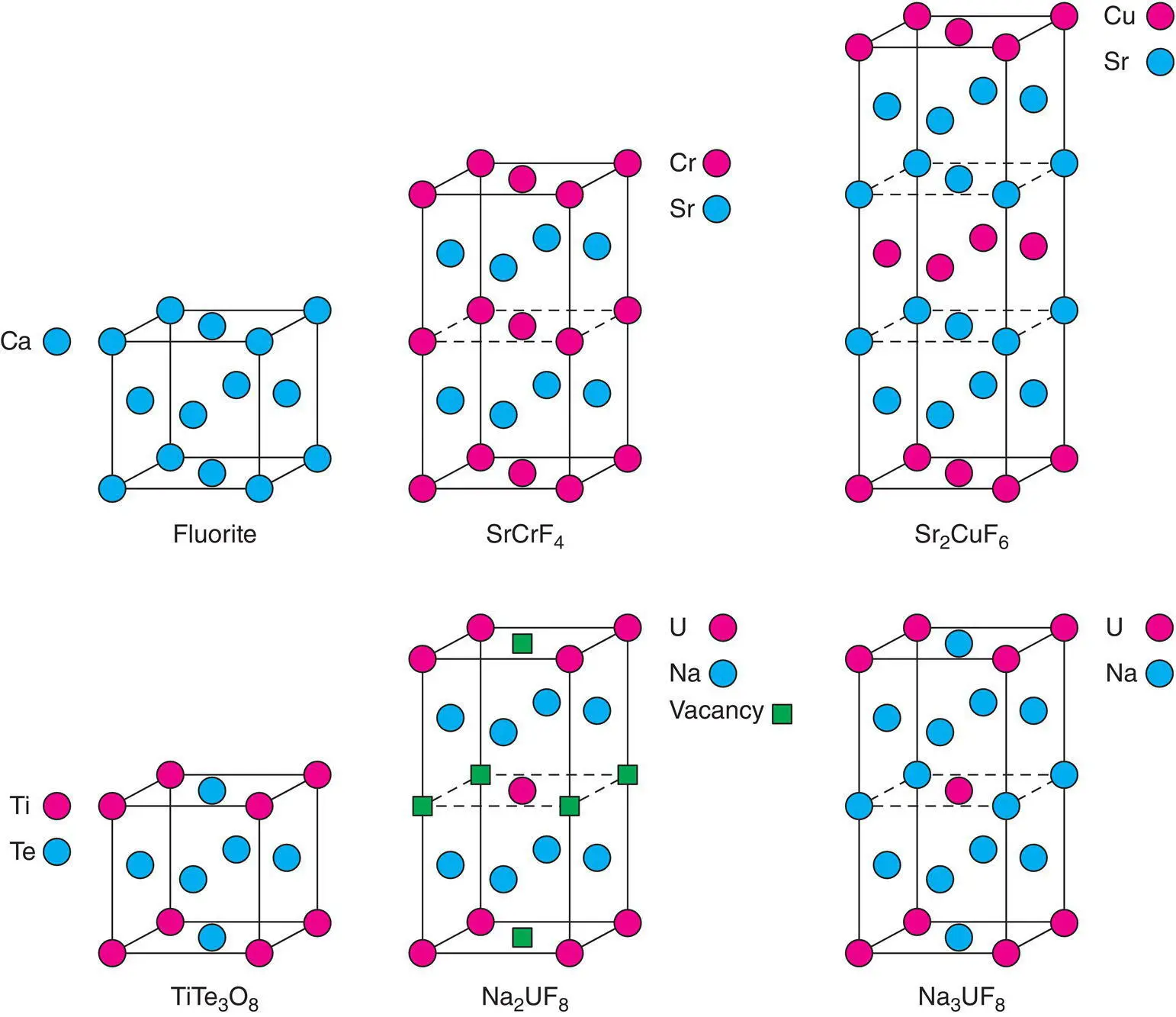
Figure 1.47 Some cation‐ordered fluorites showing cation positions relative to those in fcc fluorite.
A. F. Wells, Structural Inorganic Chemistry, Oxford University Press (2012).
The rare earth oxides RE 2O 3have structures derived from oxygen‐deficient fluorite in which the rare earth CN is reduced to 7 or 6. There are three structure types stable below 2000 °C: hexagonal A, monoclinic B and cubic C (or bixbyite) with A favoured by larger cations La to Pm, C by the smaller cations Gd to Lu and B by those of intermediate size. Some oxides are polymorphic and transform in the sequence C to B to A with increasing temperature, although most do not form all three polymorphs. Structure types and polymorphisms are summarised in Fig. 1.48(b) with part of the structure of A‐type La 2O 3in (c) showing 7‐fold coordination of La in the form of a distorted cube with one corner missing. The B structure is a monoclinic distortion of A giving rise to a mixture of 6‐ and 7‐coordinate RE cations whereas the C structure has 6‐coordinate cations. The mineral name of the C structure is bixbyite, (Fe,Mn) 2O 3and it is essentially cubic fluorite with ¼ of the oxygens missing.
Читать дальше














