A selection of compounds with the TTB structure is shown in Table 1.21. The structure is versatile since cations on the A and A′ sites can be either fully ordered, disordered or only partially occupied. In addition, there are two crystallographically distinct, octahedral B and B′ sites, which can be occupied by one species or a mixture. As with all crystal structures, it may be useful to view them in different ways and the TTB structure is shown from three different perspectives in Fig. 1.43(g).
The structure of Mo 5O 14is based on the same principles but is rather different to the TTB structure, Fig. 1.43(h) and (i). Pentagonal columns are again present and these are occupied by rows of Mo and O atoms, as shown by open and closed circles in (i). Because of the extra oxygens in the columns, Mo occupies small sites whose coordination number is reduced greatly from 15 to 7. Other pentagonal columns, and triangular columns, remain empty. In addition, new hexagonal columns are created by the array of octahedra and these remain empty in Mo 5O 14. Many other complex structures are built using similar principles, for example, NaNb 6O 15F, Nb 16W 18O 94, Bi 6Nb 34O 94and Ta 3O 7F.
Several commercially important magnetic oxides have the spinel structure. The parent spinel is MgAl 2O 4. It has ccp oxide ions with Mg 2+, Al 3+in tetrahedral and octahedral interstices, respectively. Many oxides, sulphides, and halides have the spinel structure and different cation charge combinations are possible, namely:
Similar cation combinations occur with sulphides, e.g. 2,3 as in ZnAl 2S 4and 2,4 as in Cu 2SnS 4. With halides, cations are limited to charges of 1 and 2, in order to give an overall cation:anion ratio of 3:4, e.g. Li 2NiF 4.
MgAl 2O 4has a large, cubic unit cell with a = 8.08 Å. The cell contents are eight formula units ( Z = 8) corresponding to ‘Mg 8Al 16O 32’. In the ccp oxide array, half of the octahedral sites are occupied by Al and one‐eighth of the tetrahedral sites, both T +and T –, by Mg.
The unit cell of the spinel structure is a large cube, eight times (i.e. 2 × 2 × 2) the size of a typical face centred cube, and it is almost impossible to give a comprehensible 3D drawing of the complete unit cell and its contents. We can, however, easily understand how the structure arises and draw structural fragments to illustrate certain features.
Writing the general formula of a normal spinel as A tetB 2 octO 4, let us first consider the B 2 octO 4part. This is simply the rock salt structure with ccp O 2–ions but with only alternate octahedral sites occupied by B cations. Two such rock salt‐derivative subcells are shown in Fig. 1.44(a) and (b) (each subcell forms one octant of the spinel unit cell); the bottom face of the subcell in (a) is the same as the top face of the subcell in (b). Oxygens are shown at corners and face centres (as in Fig. 1.24). Some octahedral positions are shown as occupied (solid circles) but alternate ones in any of the three unit cell directions are empty (small squares).
The base of the spinel unit cell is shown in (c); it is formed by the base of (b), dashed, and that of three adjacent subcells. The alternating, empty–occupied–empty sequence of octahedral sites is clearly seen and also occurs similarly in the third dimension (a, b).
To complete the structure, we need to locate the tetrahedral A cations. In an fcc anion array, Fig. 1.24, the eight tetrahedral sites, 5–12, are located as shown. These tetrahedral sites are equidistant from oxygen atoms but also from the octahedral cation sites (e.g. distances 9–A and 9–3 in Fig. 1.24 are equal). Cation–cation repulsions do not allow adjacent tetrahedral and octahedral sites to be occupied simultaneously (this would be equivalent to tetrahedra and octahedra sharing a common face). We therefore need to find those tetrahedral sites for which all four neighbouring octahedral sites are empty. In Fig. 1.44(a), all of the tetrahedral sites (5–12 in Fig. 1.20) have at least one neighbouring octahedral cation and so none of the tetrahedral sites in this octant is occupied. The octant immediately below (or above) that in (a) is shown in (b); note that its top face is the same as the bottom face in (a). In this octant, two of the tetrahedral sites (positions 8 and 9 in Fig. 1.24) have no octahedral cation neighbours and, therefore, both of these contain an A cation. Taking the spinel structure as a whole, the eight octants fall into two structural groups, as represented by (a) and (b). The A cations are located in the four octants of type (b).
The two types of octant are arranged such that they alternate in any of the three unit cell directions. They are, in fact, arranged in exactly the same way as anions and cations in the rock salt structure, Fig. 1.44(d). A projection of the unit cell onto one face, showing cation positions only, is shown in (e). The orientation is exactly the same as in (b) and (c) but now atom heights, above the plane of the paper, are given as multiples of c /8. Octahedral positions are solid circles; occupied tetrahedral positions are represented by A. You should be able to see that the octahedral atoms O′ in the base (height 0) are the same as in (b) and (c). An alternative view of the structure of MgAl 2O 4spinel is shown in (f). It consists of a framework of edge‐sharing AlO 6octahedra with isolated MgO 4tetrahedra inside channels formed by the octahedral array.
The above description of the spinel structure is somewhat idealised. In practice, the anions are displaced slightly from their corner and face centre positions. The degree of displacement varies slightly from compound to compound, which means that the bond lengths to A and B cations can change so as to fit best those required by the particular A and B ions. The origin of the spinel structure is usually shifted to coincide with a tetrahedral A cation. We need not pursue this any further as it may lead to confusion, but it does lead to alternative descriptions of the spinel structure (e.g. the A cations alone are arranged in the same way as the carbon atoms in the diamond structure!).
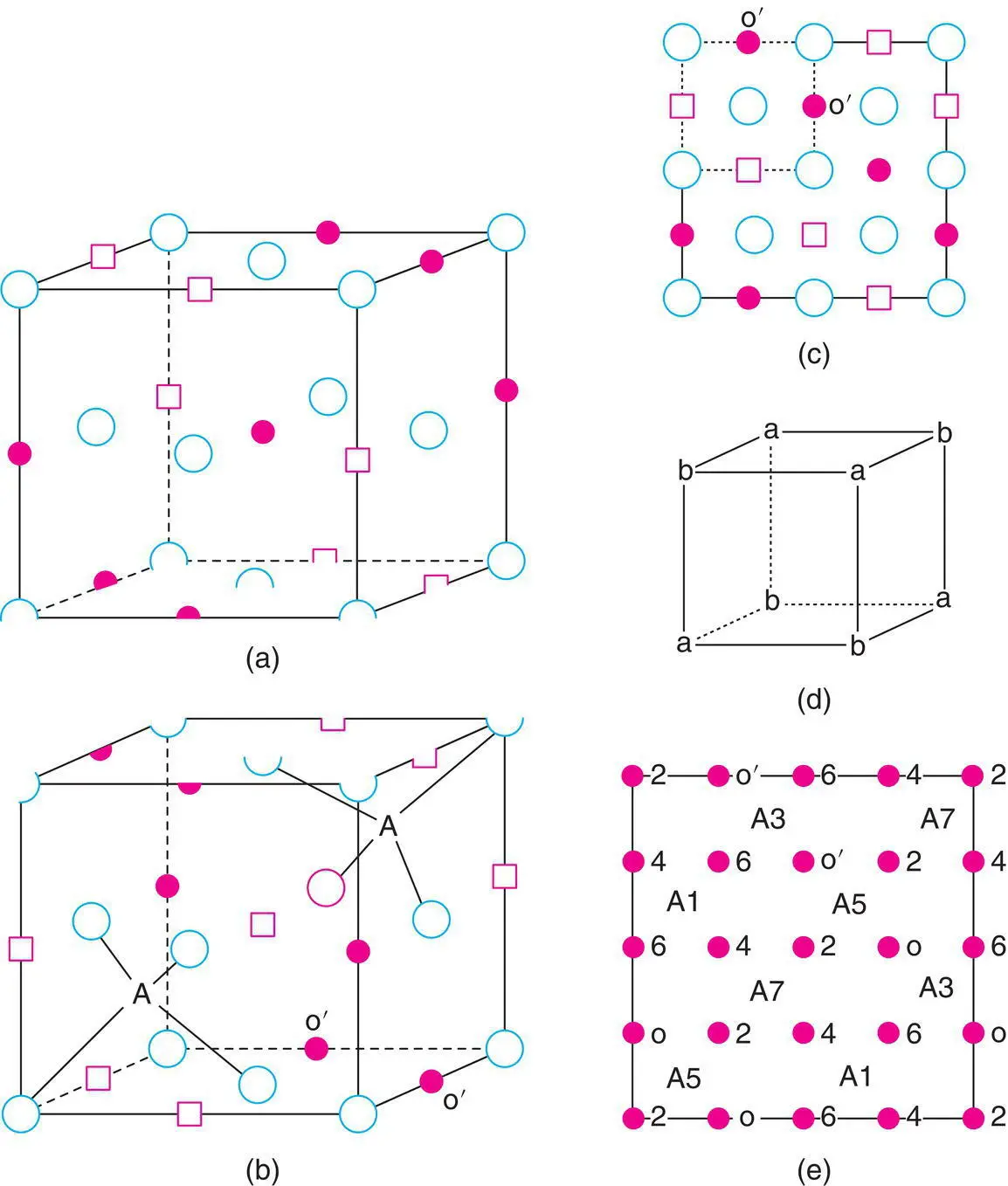
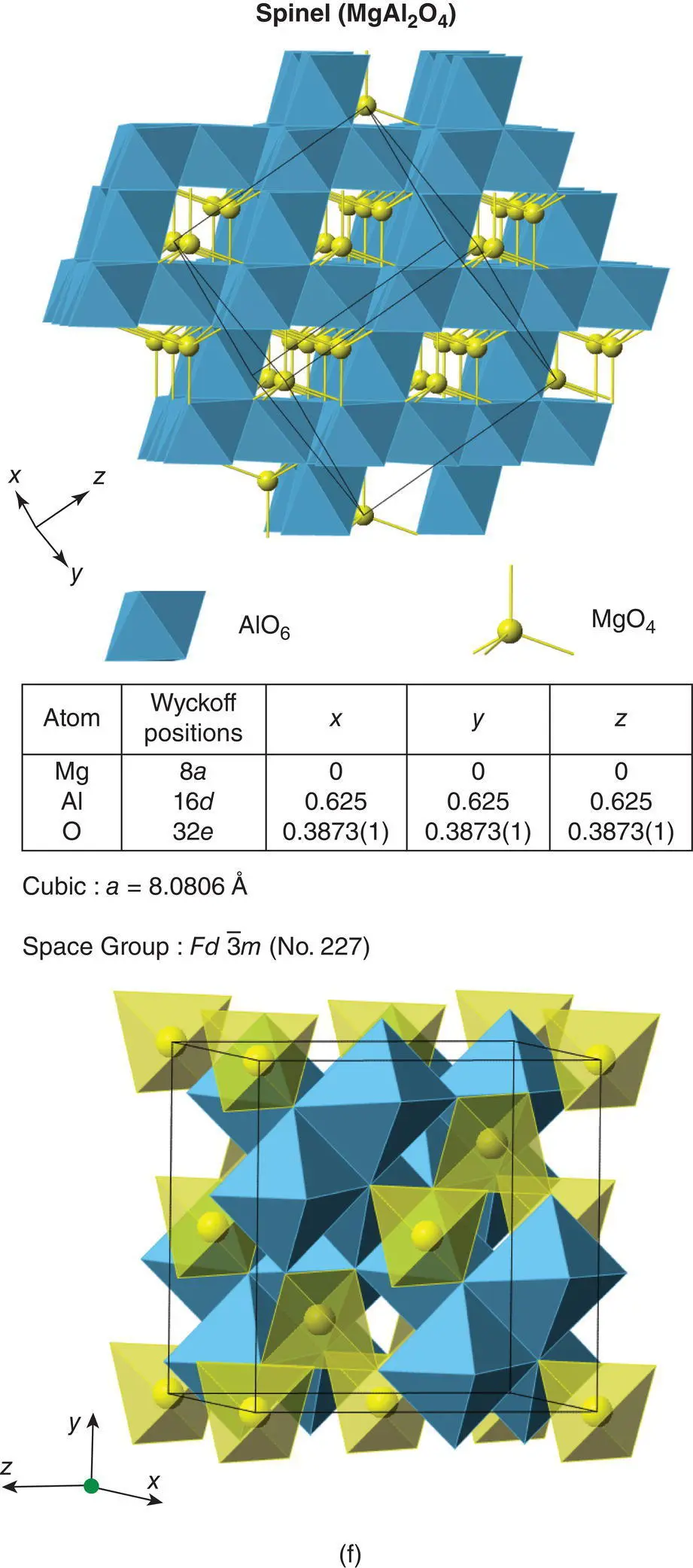
Figure 1.44 Representative parts of the spinel structure. (a) One octant of the unit cell showing oxygens at corner and face centres, empty (□) and occupied (•) octahedral sites. (b) A second octant, underneath the one in (a), showing in addition the occupation of two tetrahedral sites, A. (c) One face of the cubic unit cell of the spinel structure. The dashed part coincides with the base of the subcell shown in (b). (d) Alternating arrangement of the two types of octant (a) and (b). (e) Cation positions in spinel. Numbers refer to fractional heights, as multiples of c/8. Octahedral cation sites O′ are also shown in (b) and (c). (f) Two perspectives of the spinel structure of MgAl2O4 showing a framework of AlO6 octahedra with MgO4 tetrahedra in channel sites.
A complicating factor in some spinel structures is that the cation distribution may vary. Two extreme types of behaviour may be distinguished. In normal spinels, the cations occupy sites given by the formula
Читать дальше