1.17.4 Caesium chloride (CsCl)
The unit cell of CsCl is shown in Fig. 1.36. It is a primitive cube, containing Cl at corners and Cs at the body centre, or vice versa (note that it is not body centred cubic since there are different ions at corner and body centre positions). The coordination numbers of both Cs and Cl are eight with interatomic distances of 0.866 a , Table 1.11. The CsCl structure is not cp . In a cp structure, each anion has 12 other anions as next nearest neighbours whereas in CsCl, each Cl has only six Cl –ions as next nearest neighbours (arranged octahedrally). Some compounds with the CsCl structure are given in Table 1.14. They fall into two groups, halides of large monovalent elements and a variety of intermetallic compounds.
Lanthanum hexaboride, LaB 6, has the CsCl structure in which Cl is replaced by the octahedral cluster anion B 6 3−. LaB 6has a low work function (i.e. it ionises easily) of 2.5 eV, very high electron emissivity, metallic conductivity and is stable in vacuum. These properties enable it to be used as a hot cathode in the electron gun in electron microscopes: advantages are that it has higher brightness and longer lifetime than traditional tungsten filament cathodes.
Although CsCl is not a cp structure, there is a link between it and the fluorite structure, which can be described as a primitive cubic array of anions with cations in alternate cube body centres, Fig. 1.34; in CsCl, all body centres are occupied.
Table 1.14 Some compounds with the CsCl structure
| Compound |
a /Å |
Compound |
a /Å |
Compound |
a/ Å |
Compound |
a/ Å |
| CsCl |
4.123 |
NH 4Br |
4.0594 |
CuPd |
2.988 |
AlNi |
2.881 |
| CsBr |
4.286 |
TlCl |
3.8340 |
AuMg |
3.259 |
LiHg |
3.287 |
| CsI |
4.5667 |
TlBr |
3.97 |
AuZn |
3.19 |
MgSr |
3.900 |
| CsCN |
4.25 |
TlI |
4.198 |
AgZn |
3.156 |
|
|
| NH 4Cl |
3.8756 |
CuZn |
2.945 |
LiAg |
3.168 |
|
|
1.17.5 Other AX structures
There are five main AX structure types, rock salt, CsCl, NiAs, sphalerite and wurtzite, each of which is found in a large number of compounds. There are also several less common AX structures. Some are distorted variants of one of the main structure types, e.g.:
1 FeO at low temperatures, <90 K, has a rock salt structure with a slight rhombohedral distortion (the α angle is increased from 90 to 90.07° by a slight compression along one threefold axis). This rhombohedral distortion is associated with magnetic ordering in FeO at low temperatures (see Chapter 9).
2 TlF has a rock salt‐related structure in which the fcc cell is distorted into a face centred orthorhombic cell by changing the lengths of all three cell axes by different amounts. The distortion arises because, in this structure, Tl+ (Xe core) 4f145d106s2 is a non-spherical cation exhibiting a stereochemically-active inert (or lone) pair effect.
3 NH4CN has a distorted CsCl structure (as in NH4Cl) in which the CN– ions do not assume spherical symmetry but are oriented parallel to face diagonals. This distorts the symmetry to tetragonal and effectively increases a relative to c.
Other AX compounds have completely different structures, e.g.:
1 Compounds of the d 8 ions, Pd and Pt (in PdO, PtS, etc.), often have a square planar coordination for the cation, associated with the Jahn-Teller effect; d 9 ions also show this effect, e.g. Cu in CuO.
2 Compounds of heavy p‐block atoms in their lower oxidation states (e.g. Tl+, Pb2+, Bi3+) often have distorted polyhedra in which the cation exhibits the inert pair effect, as with TlF, above. Thus, in PbO and SnO the M2+ ion has four O2– neighbours to one side giving a square pyramidal arrangement, Fig. 3.14. InBi is similar with Bi3+ showing the inert pair effect and irregular coordination.
1.17.6 Rutile (TiO 2), cadmium iodide (CdI 2), cadmium chloride (CdCl 2) and caesium oxide (Cs 2O)
The title structures, together with fluorite, represent the main AX 2structure types. The unit cell of rutile is tetragonal a = b = 4.594 Å, c = 2.958 Å, and is shown in Fig. 1.37(a). The Ti positions, two per cell, are fixed at the corner 0, 0, 0 and body centre 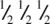 . The O positions, four per cell, have general coordinates
. The O positions, four per cell, have general coordinates 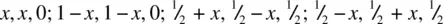 , with a variable parameter, x, whose value must be determined experimentally. Crystal structure determination and refinement gave the x values listed beneath Fig. 1.37(a) for the four oxygens in the unit cell, i.e. with x ≃ 0.3.
, with a variable parameter, x, whose value must be determined experimentally. Crystal structure determination and refinement gave the x values listed beneath Fig. 1.37(a) for the four oxygens in the unit cell, i.e. with x ≃ 0.3.
The body centre Ti at 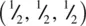 is coordinated octahedrally to six oxygens. Four of these, two at z = 0 and two at z = 1 directly above the two at z = 0, are coplanar with Ti. Two oxygens at
is coordinated octahedrally to six oxygens. Four of these, two at z = 0 and two at z = 1 directly above the two at z = 0, are coplanar with Ti. Two oxygens at 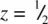 are collinear with Ti and form the axes of the octahedron. The corner Ti are also octahedrally coordinated but the orientation of their octahedra is different, Fig. 1.37(b). The oxygens are coordinated trigonally to three Ti, e.g. oxygen at 0 in (a) is coordinated to Ti at the corner, at the body centre and at the body centre of the cell below.
are collinear with Ti and form the axes of the octahedron. The corner Ti are also octahedrally coordinated but the orientation of their octahedra is different, Fig. 1.37(b). The oxygens are coordinated trigonally to three Ti, e.g. oxygen at 0 in (a) is coordinated to Ti at the corner, at the body centre and at the body centre of the cell below.
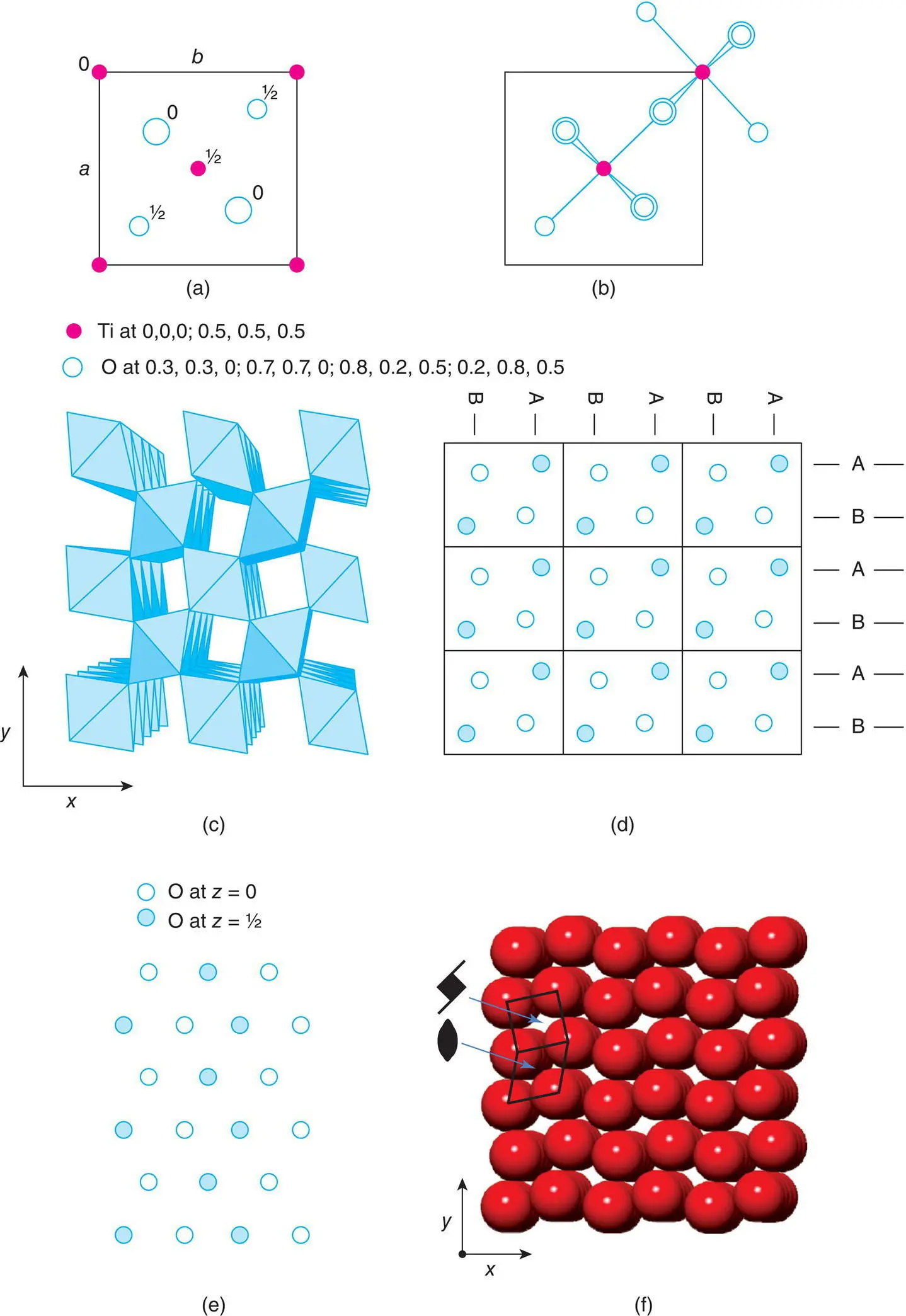
Figure 1.37 The rutile structure, TiO2: (a) the unit cell; (b) TiO6 octahedra in two orientations in the unit cell; (c) an array of octahedra in 3D; (d) oxygen atoms in zig‐zag arrangement; (e) oxygen atoms in an ideal hcp structure in projection; (f) [001] projection of the structure showing fourfold screw axes and twofold rotation axes.
The TiO 6octahedra link by sharing edges and corners to form a 3D framework. Consider the TiO 6octahedron in the centre of the cell in Fig. 1.37(b); a similar octahedron in identical orientation occurs in the cells above and below such that octahedra in adjacent cells share edges to form infinite chains parallel to c . For example, Ti at 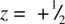 and
and 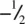 in adjacent cells are both coordinated to two oxygens at z = 0. Chains of octahedra are similarly formed by the octahedra centred at the corners of the unit cell. The two types of chains, which differ in orientation about c by 90° and which are c /2 out of step with each other, link by their corners to form a 3D framework, Fig. 1.37(c).
in adjacent cells are both coordinated to two oxygens at z = 0. Chains of octahedra are similarly formed by the octahedra centred at the corners of the unit cell. The two types of chains, which differ in orientation about c by 90° and which are c /2 out of step with each other, link by their corners to form a 3D framework, Fig. 1.37(c).
The rutile structure is also commonly described as a distorted hcp oxide array with half the octahedral sites occupied by Ti. A 3 × 3 block of unit cells is shown in Fig. 1.37(d) with only the oxygen positions marked. Corrugated cp layers occur, both horizontally and vertically. This contrasts with the undistorted hcp arrangement (e), in which the layers occur in one orientation only (horizontally).
Читать дальше

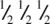 . The O positions, four per cell, have general coordinates
. The O positions, four per cell, have general coordinates 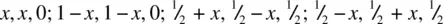 , with a variable parameter, x, whose value must be determined experimentally. Crystal structure determination and refinement gave the x values listed beneath Fig. 1.37(a) for the four oxygens in the unit cell, i.e. with x ≃ 0.3.
, with a variable parameter, x, whose value must be determined experimentally. Crystal structure determination and refinement gave the x values listed beneath Fig. 1.37(a) for the four oxygens in the unit cell, i.e. with x ≃ 0.3.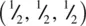 is coordinated octahedrally to six oxygens. Four of these, two at z = 0 and two at z = 1 directly above the two at z = 0, are coplanar with Ti. Two oxygens at
is coordinated octahedrally to six oxygens. Four of these, two at z = 0 and two at z = 1 directly above the two at z = 0, are coplanar with Ti. Two oxygens at 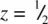 are collinear with Ti and form the axes of the octahedron. The corner Ti are also octahedrally coordinated but the orientation of their octahedra is different, Fig. 1.37(b). The oxygens are coordinated trigonally to three Ti, e.g. oxygen at 0 in (a) is coordinated to Ti at the corner, at the body centre and at the body centre of the cell below.
are collinear with Ti and form the axes of the octahedron. The corner Ti are also octahedrally coordinated but the orientation of their octahedra is different, Fig. 1.37(b). The oxygens are coordinated trigonally to three Ti, e.g. oxygen at 0 in (a) is coordinated to Ti at the corner, at the body centre and at the body centre of the cell below.
 and
and 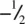 in adjacent cells are both coordinated to two oxygens at z = 0. Chains of octahedra are similarly formed by the octahedra centred at the corners of the unit cell. The two types of chains, which differ in orientation about c by 90° and which are c /2 out of step with each other, link by their corners to form a 3D framework, Fig. 1.37(c).
in adjacent cells are both coordinated to two oxygens at z = 0. Chains of octahedra are similarly formed by the octahedra centred at the corners of the unit cell. The two types of chains, which differ in orientation about c by 90° and which are c /2 out of step with each other, link by their corners to form a 3D framework, Fig. 1.37(c).










