The octahedral site in Fig. 1.35(d) is coordinated to three anions at c = 0 and three anions at 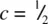 . The centre of gravity of the octahedron lies midway between these two groups of anions and has coordinates
. The centre of gravity of the octahedron lies midway between these two groups of anions and has coordinates 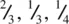 . The second octahedral site lies immediately above the octahedral site shown in (d) and has coordinates
. The second octahedral site lies immediately above the octahedral site shown in (d) and has coordinates 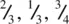 (e). The three anions at
(e). The three anions at 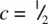 are therefore common to the two octahedra, which means that octahedral sites share opposite faces.
are therefore common to the two octahedra, which means that octahedral sites share opposite faces.
The coordination environments of the cations in wurtzite and NiAs are emphasised in Fig. 1.35(f) and (g). Zinc is shown in T +sites and forms ZnS 4tetrahedra (f), linked at their corners to form a 3D network, as in (j). A similar structure results on considering the tetrahedra formed by four Zn atoms around a S. The tetrahedral environment of S (1) is shown in (f). The SZn 4tetrahedron which it forms points down, in contrast to the ZnS 4tetrahedra, all of which point up; on turning the SZn 4tetrahedra upside down, however, the same structure results.
Comparing larger scale models of zinc blende [Fig. 1.33(b)] and wurtzite [Fig. 1.35(j)], they are clearly very similar and both can be regarded as networks of tetrahedra. In zinc blende, layers of tetrahedra form an ABC stacking sequence and the orientation of the tetrahedra within each layer is identical. In wurtzite, the layers form an AB sequence and alternate layers are rotated by 180° about c relative to each other.
The NiAs 6octahedra in NiAs are shown in Fig. 1.35(g). They share one pair of opposite faces (e.g. the face formed by arsenic ions 1, 2 and 3) to form chains of face‐sharing octahedra that run parallel to c . In the ab plane, however, the octahedra share only edges: As atoms 3 and 4 are shared between two octahedra such that chains of edge‐sharing octahedra form parallel to b . Similarly, chains of edge‐sharing octahedra form parallel to a (not shown). A more extended view of the octahedra and their linkages is shown in (k).
The NiAs structure is unusual in that the anions and cations have the same coordination number but different coordination environments. Since the cation:anion ratio is 1:1 and the Ni coordination is octahedral, As must also be six‐coordinate. However, the six Ni neighbours are arranged as in a trigonal prism and not octahedrally. This is shown for As at 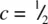 in Fig. 1.35(h), which is coordinated to three Ni at
in Fig. 1.35(h), which is coordinated to three Ni at 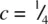 and three at
and three at 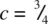 . The two sets of Ni are superposed in projection down c and give trigonal prismatic coordination for As. [Note that in a similar projection for octahedral coordination, the two sets of three coordinating atoms are staggered relative to each other, as in (e).]
. The two sets of Ni are superposed in projection down c and give trigonal prismatic coordination for As. [Note that in a similar projection for octahedral coordination, the two sets of three coordinating atoms are staggered relative to each other, as in (e).]
The NiAs structure may also be regarded as built of AsNi 6trigonal prisms, therefore, which link up by sharing edges to form a 3D array. In Fig. 1.35(i), each triangle represents a prism in projection down c . The prism edges that run parallel to c , i.e. those formed by Ni at 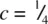 and
and 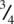 in (h), are shared between three prisms. Prism edges that lie in the ab plane are shared between only two prisms, however. In (i), the edge xy is shared between As at
in (h), are shared between three prisms. Prism edges that lie in the ab plane are shared between only two prisms, however. In (i), the edge xy is shared between As at 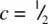 and c = 0. The structure therefore has layers of prisms arranged in an … ABABA … hexagonal stacking sequence, as shown further in (1).
and c = 0. The structure therefore has layers of prisms arranged in an … ABABA … hexagonal stacking sequence, as shown further in (1).
The NiAs structure can be described as hcp As with Ni in fully occupied octahedral interstitial sites. However, unlike the case of NaCl where Na and Cl positions are interchangeable, we cannot simply exchange Ni, As and arrive at the same structural description. If we consider the arrangement of Ni alone, it still forms cp layers but the stacking sequence along c is identical because Ni atoms are superposed in projection, Fig. 1.35(h). Since both As and Ni form cp layers, we may describe the combined layer stacking sequence parallel to c as ACBCACBC… where Ni atoms in C positions (red) separate the A and B layers of As (black).
A selection of compounds with wurtzite and NiAs structures is given in Table 1.12 and Table 1.13with values of their hexagonal cell parameters a and c . The wurtzite structure is formed mainly by chalcogenides of divalent metals and is a fairly ionic structure. The NiAs structure is more metallic and is adopted by a variety of intermetallic compounds and some transition metal chalcogenides (S, Se, and Te). The value of the ratio c / a is approximately constant in the wurtzite structures but varies considerably in compounds with the NiAs structure. This is associated with the presence of metallic bonding which arises from metal–metal interactions in the c direction, as follows. First consider the environment of Ni and As:
Each As is surrounded by (Table 1.11):6 Ni in a trigonal prism at distance 0.707a12 As, hcp arrangement, at distance a. Table 1.13Some compounds with the NiAs structureCompounda/Åc/Åc/aCompounda/Åc/Åc/aNiS3.43925.34841.555CoS3.3675.1601.533NiAs3.6025.0091.391CoSe3.62945.30061.460NiSb3.945.141.305CoTe3.8865.3601.379NiSe3.66135.35621.463CoSb3.8665.1881.342NiSn4.0485.1231.266CrSe3.6846.0191.634NiTe3.9575.3541.353CrTe3.9816.2111.560FeS3.4385.8801.710CrSb4.1085.4401.324FeSe3.6375.9581.638MnTe4.14296.70311.618FeTe3.8005.6511.487MnAs3.7105.6911.534FeSb4.065.131.264MnSb4.1205.7841.404δ′‐NbN a 2.9685.5491.870MnBi4.306.121.423PtB a 3.3584.0581.208PtSb4.1305.4721.325PtSn4.1035.4281.323PtBi4.3155.4901.272 a Anti‐NiAs structure.R. W. G. Wyckoff, Crystal Structures, Vols 1 to 6, Wiley (1971). Figure 1.36The primitive cubic unit cell of CsCl.
Each Ni is surrounded by:6 As, octahedrally, at distance 0.707a2 Ni, linearly, parallel to c, at distance 0.816a (i.e. c/2)6 Ni, hexagonally, in ab plane at distance a.
The main effect of changing the value of the c / a ratio is to alter the Ni–Ni distance parallel to c . Thus, in FeTe, c/a = 1.49, and the Fe–Fe distance is reduced to 0.745 a [i.e. 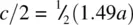 ], thereby bringing these Fe atoms into close contact and increasing the metallic bonding in the c direction. Simple quantitative calculations of the effect of changing the c/a ratio are difficult to make since it is not readily possible to distinguish between, for example, an increase in a and a decrease in c , either of which could cause the same effect on the c/a ratio.
], thereby bringing these Fe atoms into close contact and increasing the metallic bonding in the c direction. Simple quantitative calculations of the effect of changing the c/a ratio are difficult to make since it is not readily possible to distinguish between, for example, an increase in a and a decrease in c , either of which could cause the same effect on the c/a ratio.
Читать дальше

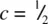 . The centre of gravity of the octahedron lies midway between these two groups of anions and has coordinates
. The centre of gravity of the octahedron lies midway between these two groups of anions and has coordinates 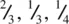 . The second octahedral site lies immediately above the octahedral site shown in (d) and has coordinates
. The second octahedral site lies immediately above the octahedral site shown in (d) and has coordinates 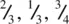 (e). The three anions at
(e). The three anions at 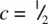 are therefore common to the two octahedra, which means that octahedral sites share opposite faces.
are therefore common to the two octahedra, which means that octahedral sites share opposite faces.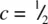 in Fig. 1.35(h), which is coordinated to three Ni at
in Fig. 1.35(h), which is coordinated to three Ni at 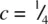 and three at
and three at 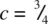 . The two sets of Ni are superposed in projection down c and give trigonal prismatic coordination for As. [Note that in a similar projection for octahedral coordination, the two sets of three coordinating atoms are staggered relative to each other, as in (e).]
. The two sets of Ni are superposed in projection down c and give trigonal prismatic coordination for As. [Note that in a similar projection for octahedral coordination, the two sets of three coordinating atoms are staggered relative to each other, as in (e).]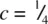 and
and 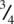 in (h), are shared between three prisms. Prism edges that lie in the ab plane are shared between only two prisms, however. In (i), the edge xy is shared between As at
in (h), are shared between three prisms. Prism edges that lie in the ab plane are shared between only two prisms, however. In (i), the edge xy is shared between As at 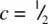 and c = 0. The structure therefore has layers of prisms arranged in an … ABABA … hexagonal stacking sequence, as shown further in (1).
and c = 0. The structure therefore has layers of prisms arranged in an … ABABA … hexagonal stacking sequence, as shown further in (1).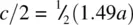 ], thereby bringing these Fe atoms into close contact and increasing the metallic bonding in the c direction. Simple quantitative calculations of the effect of changing the c/a ratio are difficult to make since it is not readily possible to distinguish between, for example, an increase in a and a decrease in c , either of which could cause the same effect on the c/a ratio.
], thereby bringing these Fe atoms into close contact and increasing the metallic bonding in the c direction. Simple quantitative calculations of the effect of changing the c/a ratio are difficult to make since it is not readily possible to distinguish between, for example, an increase in a and a decrease in c , either of which could cause the same effect on the c/a ratio.










