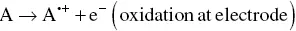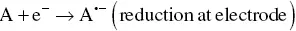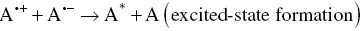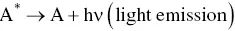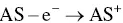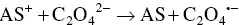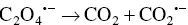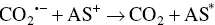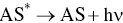It is clear that a big effort has been demanded to obtain materials or molecules that could greatly improve the ECL efficiency in order to ameliorate in parallel to the efficiency of sensing devices, which use this technique as a detection module. Recently, the rising interest of our group for ECL of platinum(II) complexes has enlightened their ability to produce high ECL emission through self‐assembly [28], connecting this impressive luminescence with the phenomenon AIE, explored in this book. Therefore, AI‐ECL has been coined to explain why aggregation can improve ECL, or in some cases generate it. From this first revealed phenomenon, several groups have tested AIE luminophores with ECL measurements to study the possibility of an AI‐ECL and its potential applications. This chapter will then focus on describing the phenomenon, the mechanisms, and the most important examples published by different research groups in the last three years.
4.1.1 Mechanisms of AI‐ECL
There is not a particular class of mechanisms for AI‐ECL, therefore, ECL classic mechanisms can be used to explain this phenomenon [1, 29]. In ECL, the excited state responsible for the emission of the photon, is generated from the reaction of intermediates generated electrochemically. Indeed, ECL shares feature with both chemiluminescence and electroluminescence, since light emission is ultimately initiated and controlled by application of a potential at an electrode.
In a basic ECL experiment, a potential scan from anodic to cathodic part is performed to a solution of [Ru(bpy) 3] 2+. A red luminescence develops at the working electrode at the oxidation and reduction potentials of the complex. The same red emission can be obtained when an amine is added to the solution. Upon anodic scan, it is possible to collect light right in front of the electrode surface.
The two cases outlined represent the two main approaches used in the development of ECL applications, dubbed the ion annihilation ( Figure 4.2) and the coreactant approaches ( Figure 4.3). The latter can be further classified into oxidative‐reductive ECL or reductive‐oxidative ECL.
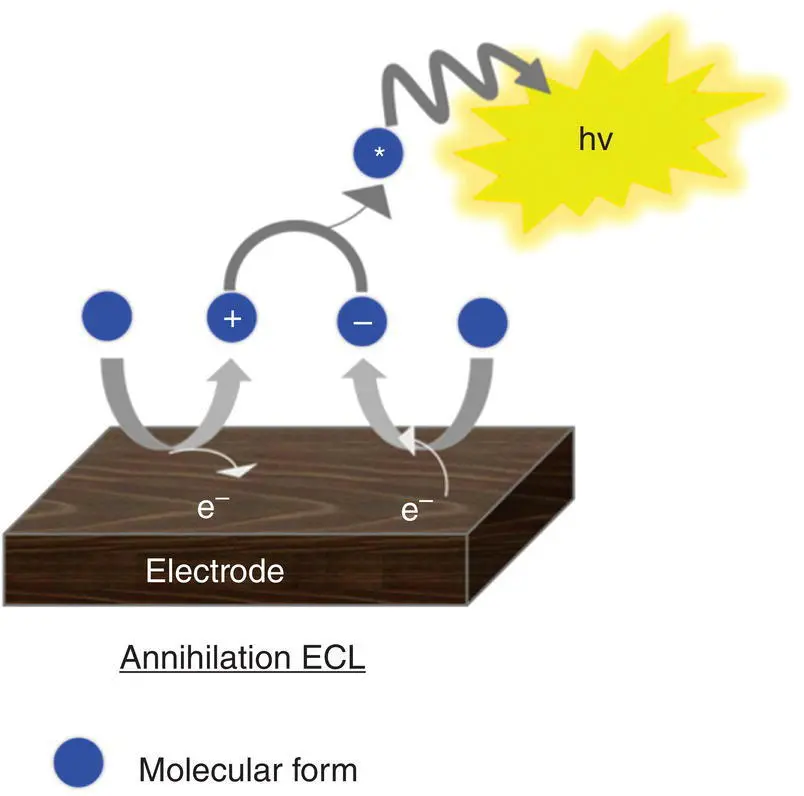
Figure 4.2 Schematic diagram describing the electron transfer reactions responsible for emission during annihilation ECL.
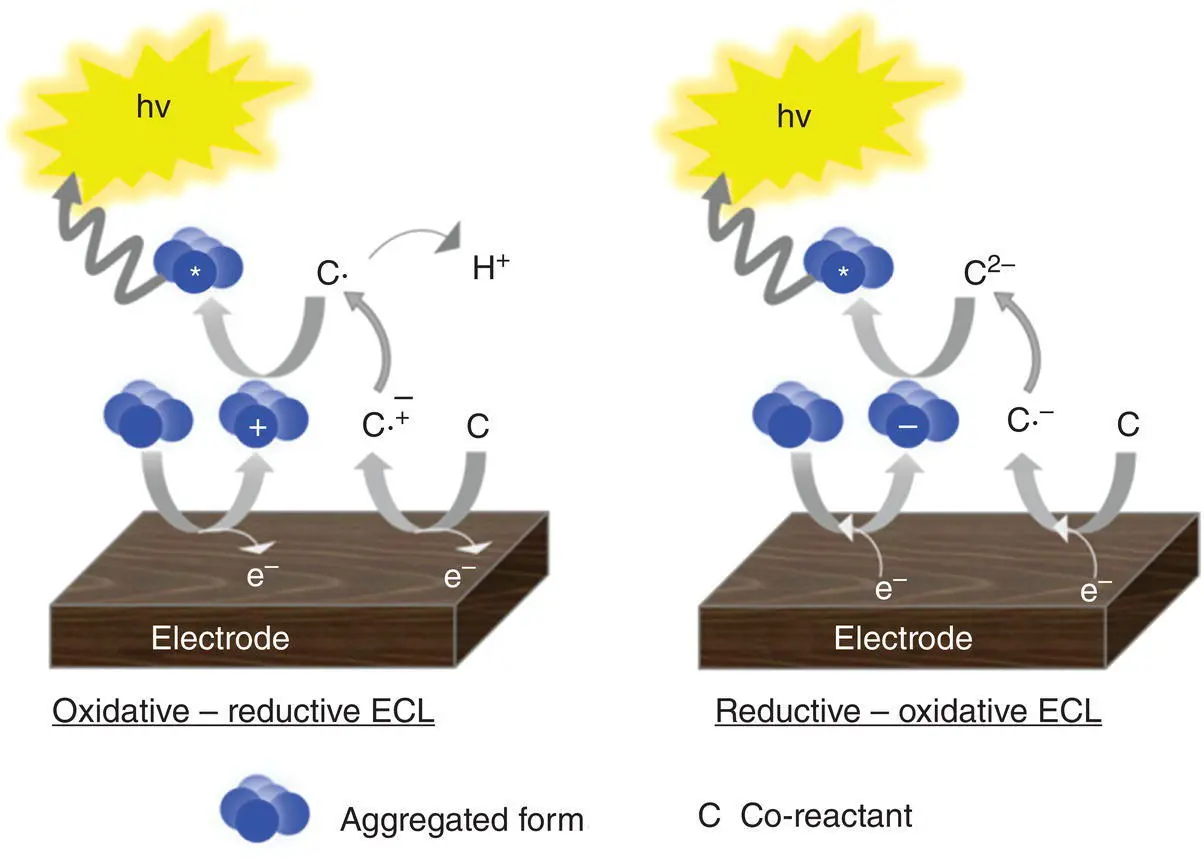
Figure 4.3 Schematic diagram describing the electron transfer reactions responsible for emission during a coreactant ECL reaction: on the left the oxidative‐reductive pathway, on the right side the reductive–oxidative pathway.
In the ECL timeline, the ion annihilation approach for the generation of excited states was the first one explored due to the relative simplicity of the system involved. One single species, A, is both reduced and oxidized (to A +and A −, respectively) at an electrode, by alternate pulsing of the electrode potential, according to Equations 4.1and 4.2[3, 30]. These species react in the Nernst diffusion layer over the electrode according to Equation 4.3, to yield an excited state A* along with a molecule in its ground state A.
(4.1) 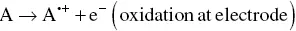
(4.2) 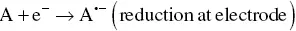
(4.3) 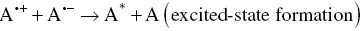
(4.4) 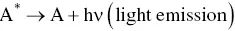
Most annihilation ECL materials are organometallic complexes, and only one report has shown an AI‐ECL generated by annihilation pathway, which consists of a donor‐acceptor conjugated polymer dot (Pdot) and it will be elucidated in Section 4.3.2[31].
All the other reports on AI‐ECL, involve the coreactant mechanisms, which not only are easier to operate but also cover the major research direction of possible applications in biosensing. Indeed, the ECL coreactant mechanism is the basis of all commercially available instruments [32].
Considering one potential step generation, coreactant ECL shows several advantages over annihilation ECL. First, there is no need for a wide potential window so other solvents with a narrow potential window and aqueous solution can be also used. Further, there is no need of rigorously purified and deoxygenated solvents because oxygen and water quenching are less efficient. Thus, a reaction can be carried out in the air. Finally, the use of coreactant makes ECL possible even for some fluorophores that have only a reversible electrochemical oxidation or reduction, while annihilation ECL, in general, requires both of them.
A coreactant is a species that upon electrochemical oxidation or reduction undergoes fast chemical decomposition to form a high‐energy reducing or oxidizing intermediate. The latter can react with an oxidized or reduced luminophore to generate excited states ( Figure 4.3).
The oxidative‐reductive coreactant mechanism finds one of the best candidates in oxalates [33], as discovered by Bard and co‐workers, and extensively studied by many research groups [34–38]. AI‐ECL can also be generated by using oxalate in aqueous solution, as explained during the first discovery by our group and in subsequent reports [27, 28, 39]. Oxidation of oxalate would produce oxalate anion radicals (C 2O 4 ∙−), which is then followed by a chemical decomposition to form a highly reducing intermediate (CO 2 ∙−, E °= 1.9 V vs. NHE) which react with the oxidized AS at the electrode to generate ECL emission. The corresponding mechanism is described in Equations 4.5– 4.11, and shown in on the left side of Figure 4.3.
(4.5) 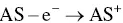
(4.6) 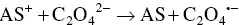
(4.7) 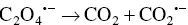
(4.8) 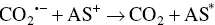
(4.9) 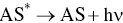
This is a typical case of an electron transfer chemical reaction (EC′) reaction [40], which has been extensively discussed by Bard et al. [33]. By applying the anodic potential, the AS is first oxidized to AS +at the electrode surface. This cation is then capable of oxidizing C 2O 4 2−in the diffusion layer close to the electrode surface to form an oxalate radical anion C 2O 4 •−, which decomposes to a highly reducing anion radical CO 2 •−and carbon dioxide. The excited state AS* can be obtained by direct reaction between CO 2 •−and the oxidized AS +.
Читать дальше