114 114 Liu B, Zhang R (2017). Aggregation induced emission: concluding remarks. Faraday Discus. 196(0): 461–72.
4 Aggregation‐induced Electrochemiluminescence
Serena Carrara1,2
1 Department of Chemistry & Physics, La Trobe Institute for Molecular Science, La Trobe University, Melbourne, VIC, Australia
2 Aix Marseille Université, CNRS, CINAM, Marseille, France
Electrogenerated chemiluminescence (ECL) is the light generated and controlled by the application of a potential at an electrode surface through an electron transfer reaction.
Recently, the enthusiastic phenomenon of aggregation‐induced electrogenerated chemiluminescence (AI‐ECL) has come to light, examining a class of supramolecular assemblies and nanostructure that can emit stronger ECL than the corresponding unassembled system can do. This new concept brings together electrochemical and photophysical properties of aggregates and new fascinating and unexplored mechanisms for its generation.
The findings can lead to a new generation of bright emitters that can be used as ECL labels in immunoassays and different biosensors, where the change in the packing of the aggregated systems (ASs) can be monitored by an off/on ECL signal or even different colors.
The following chapter outlines the general principle and mechanisms of AI‐ECL together with the new emerging emitters in the field of metal complexes, organic molecules, and materials. Then, an overview of the main applications will be presented, focusing mostly on bioanalysis.
4.1 Introduction: From Electrochemiluminescence to AI‐ECL
ECL is literally a light generated at the electrode surface through a heterogeneous electron transfer that forms an excited state [1, 2]. It is a means of converting electrical energy into radiative energy. Comparing to other ways of generation of excited states, ECL has many advantages like spatial and temporal control, since the light is obtained onto the electron surface and upon an applied voltage. Also, it does not require a light source for the excitation process, allowing the use of a more sensitive set‐up convenient for sensing application. Its main importance in terms of applications lies in its usefulness for analytical detection, offering superior detection limits to fluorescence without the complexity of chemiluminescence methods. Indeed, all these benefits have conducted the use of this technique in industrial application, especially in the field of diagnostics, being up to now the most used one from Roche Diagnostics for immunoassays tests of biological markers [3–5]. The first investigations of ECL emission were done on rubrene and related compounds in the 1960s [6, 7]. But it is from the ECL studies on tris(2,2′‐bipyridine)ruthenium(II), [Ru(bpy) 3] 2+, that this phenomenon took hold on stage creating a bridge between photophysics and electrochemistry that has not had any point of return [8–10].
Ru(bpy) 3 2+was widely explored for fundamental and application purposes because of its chemical, photochemical, and electrochemical stability [2, 3]. Its structure is represented in Figure 4.1, together with its cyclic voltammetry. The latter shows the various redox processes, with a one‐electron oxidation relative to the metal center Ru II/Ru IIIand three closely spaced one‐electron ligand reductions. All the processes result reversible with a similar magnitude of the cathodic and anodic peaks.
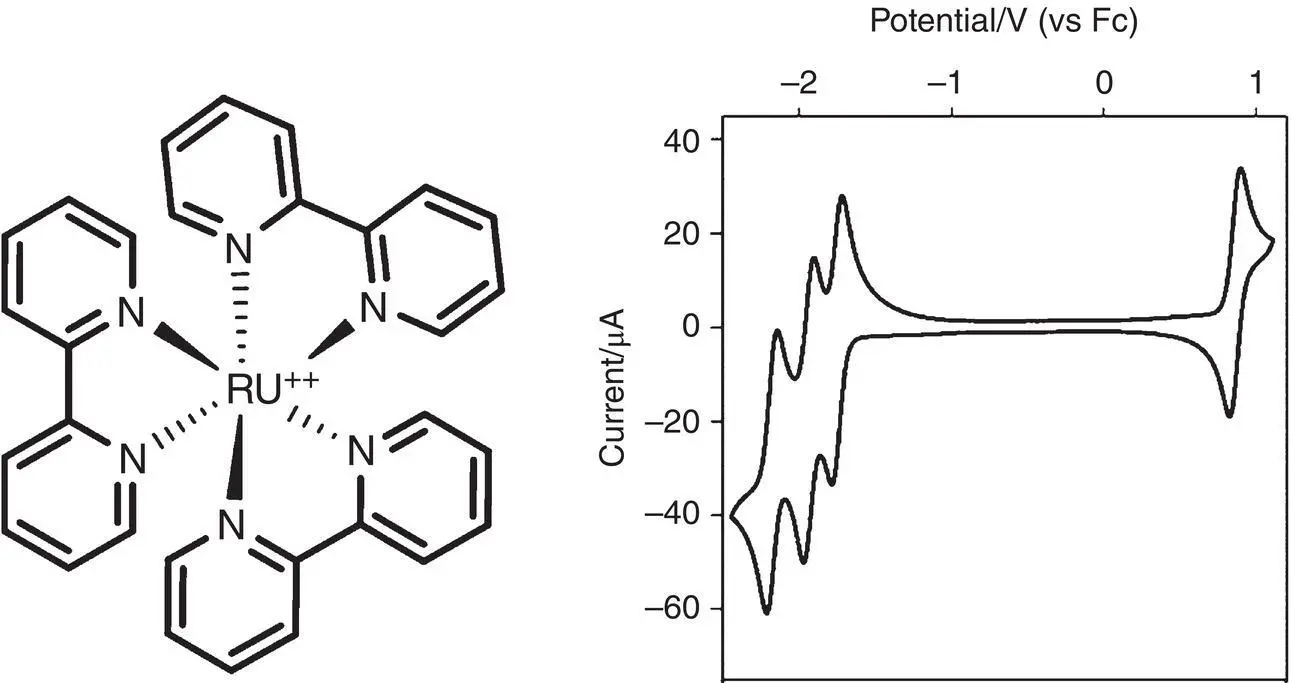
Figure 4.1 [Ru(bpy) 3] 2+structure and its cyclic voltammetry in acetonitrile.
Thanks to all these excellent qualities, this metal complex has been the main character for studies on mechanisms regulating the formation of excited states via electrochemistry. Two main mechanisms have been defined and studied during the years: annihilation and coreactant.
Annihilation ECL happens when the molecule is oxidized and reduced at the electrode surface applying an alternated potential in short time scales. These two oxidation states react together via electron transfer process from the reduced species to the oxidized one obtaining, therefore, as products one molecule at the excited state and one at the ground state [1]. Such mechanism is mainly performed in rigorously purified and deoxygenated nonaqueous media, because the available potential range in water is too narrow to generate the required energetic precursors. For this intent, acetonitrile, dimethyl‐sulfoxide, or methylene‐chloride are the most employed, with tetra n ‐butylammonium perchlorate or tetraethylammonium perchlorate as a supporting electrolyte. Water and oxygen are harmful to these experiments because they can quench ECL. Thus, cells and electrodes have to be constructed to allow transfer of solvent and degassing on high‐vacuum line or in an inert atmosphere (glove boxes) [2].
In contrast, coreactant ECL requires the presence of another species, so‐called coreactant, which helps the emitter in the generation of the excited state. Instead of alternating the potential pulse, the system undergoes only an oxidation or reduction, which transforms the coreactant into a highly unstable species that decompose producing a powerful oxidant or reducing agent [11, 12]. Therefore, this agent will excite by a high‐energy electron transfer with the luminophore, which will emit light in the end.
Depending on the case, if the luminophore is first oxidized at the electrode surface, and then reduced by strongly reducing intermediate, the corresponding ECL is called oxidative‐reduction ECL. On the other way around, if a cathodic potential is applied and the reduced luminophore is oxidized by a strong oxidizing intermediate, the corresponding ECL is called reductive‐oxidation ECL [2]. These mechanisms will be investigated in detail later in Section 4.2.
It is noteworthy to say that since the initial work on it by Bard in 1972 [8], there have been over 3700 papers published concerning the ECL of ruthenium complexes, a considerable share of the field representing at least 70% of all ECL papers.
Together with the study of different mechanisms involving Ru(bpy) 3 2+, in the past decades, there has been an intense interest in finding new emitters which could improve the ECL efficiency and could emit different colors. Among them, we can find iridium‐based metal complexes, organic molecules, and nanomaterials.
In general, cyclometalated Ir(III) complexes have unique photophysical properties comparing to Ru(II) complexes, such as excellent color tuning and relatively longer lifetimes, and higher quantum yields [13–16]. An important breakthrough in the use of cyclometalated Ir(III) for ECL was made by Kim et al., who reported a series of bis‐cyclometalated complexes, such as [Ir(ppy) 2(bpy)] +and [Ir(ppy) 2(phen)] +that were giving superior intensities than [Ru(bpy) 3] 2+by coreactant or annihilation [17].
Although not as important as organometallic emitters, organic electrochemiluminophores have continued to be of some importance for the field. 9,10‐diphenylanthracene (DPA) and its derivatives are considered the gold standard for organic ECL luminophores due to their remarkable luminescence properties in organic media [18, 19]. Their use in aqueous systems for biological application was pursued by Bard et al., who produced dispersed nanoparticles of DPA derivatives in an aqueous solution [20]. Unfortunately, they produced a rather weak ECL emission due to their slow diffusion toward the electrode surface.
In addition, after the first report of ECL of silicon nanocrystals in 2002 [21], a series of nanomaterials with various compositions, sizes, and shapes has been examined [22, 23]. These include nanoparticles and nanotubes prepared from metals [24], semiconductor [25], carbon [26], or polymeric species [27].
Читать дальше













