X-Ray Fluorescence in Biological Sciences
Здесь есть возможность читать онлайн «X-Ray Fluorescence in Biological Sciences» — ознакомительный отрывок электронной книги совершенно бесплатно, а после прочтения отрывка купить полную версию. В некоторых случаях можно слушать аудио, скачать через торрент в формате fb2 и присутствует краткое содержание. Жанр: unrecognised, на английском языке. Описание произведения, (предисловие) а так же отзывы посетителей доступны на портале библиотеки ЛибКат.
- Название:X-Ray Fluorescence in Biological Sciences
- Автор:
- Жанр:
- Год:неизвестен
- ISBN:нет данных
- Рейтинг книги:5 / 5. Голосов: 1
-
Избранное:Добавить в избранное
- Отзывы:
-
Ваша оценка:
- 100
- 1
- 2
- 3
- 4
- 5
X-Ray Fluorescence in Biological Sciences: краткое содержание, описание и аннотация
Предлагаем к чтению аннотацию, описание, краткое содержание или предисловие (зависит от того, что написал сам автор книги «X-Ray Fluorescence in Biological Sciences»). Если вы не нашли необходимую информацию о книге — напишите в комментариях, мы постараемся отыскать её.
Discover a comprehensive exploration of X-ray fluorescence in chemical biology and the clinical and plant sciences X-Ray Fluorescence in Biological Sciences: Principles, Instrumentation, and Applications
X-Ray Fluorescence in Biological Sciences: Principles, Instrumentation, and Applications
X-Ray Fluorescence in Biological Sciences — читать онлайн ознакомительный отрывок
Ниже представлен текст книги, разбитый по страницам. Система сохранения места последней прочитанной страницы, позволяет с удобством читать онлайн бесплатно книгу «X-Ray Fluorescence in Biological Sciences», без необходимости каждый раз заново искать на чём Вы остановились. Поставьте закладку, и сможете в любой момент перейти на страницу, на которой закончили чтение.
Интервал:
Закладка:
6.3 Determination of Lead Concentrations in Human Whole Blood Using EDXRF Technique with Special Emphasis on Evaluating Association of Blood Lead Levels with Autism Spectrum Disorders (ASD)
6.3.1 Background
Autism spectrum disorders is an emerging and increasingly relevant point of social concern. ASD is a range of potentially devastating childhood conditions associated with environmental, genetic, and epigenetic factors. Some of these effects appear to be transgenerational. ASD is a kind of neurodevelopment disorder that can produce features such as impaired psychosocial and adaptive skills functioning, as well as lack of social interaction, poor communication skills, and compulsive patterns of activity [9] are also reported regarding ASD. It is thought that autism is initiated by disruption of normal neurobiological mechanisms during the prenatal period, accompanied by strong genetic components, but nongenetic factors are likely involved as well with increased prevalence.
A combination of genetic susceptibility and exposure to environmental toxins at critical periods during brain development has recently been hypothesized as the origin of autism [10] and several studies have observed elevated levels of heavy metals and essential minerals among children diagnosed with ASD [9]. Johnson and Myers [11] suggested that environmental exposure to heavy metals and essential minerals may act as a central nervous system teratogen in early gestational life. Among the toxic heavy metals, Pb is of particular concern as an undesirable contaminant originating from a variety of common sources such as motor vehicles, lead paint, contaminated soil etc. Pb can be ingested through air, drinking water, food etc. Developing fetuses and children are more sensitive to Pb exposure compared to adults because of the relative immaturity of the blood–brain barrier, increased gastrointestinal absorption, and the prevalence of hand‐to‐mouth behaviors. Hence the possibility of Pb exposure leading to the development of ASD in children is of greater concern to children than adults.
Many studies from across the world support the association between exposure to Pb and ASD. But different, conflicting [12] opinions are also available and must be taken into consideration. Adams et al. [13] conducted a study where they compared 55 children with autism ages 5–16 years to 44 control subjects of similar age and gender and reported that the autism group had significantly higher levels of Pb in their red blood cells and higher urinary levels of lead, thallium, tin, and tungsten. These are toxic metals that can impair brain development and function, and also interfere with the normal functioning of other bodily organs and systems. Exposure to Pb can pose a substantial risk to nervous, immune, renal, skeletal, and hematopoietic systems of both adult and children. Neurological, physiological, and behavioral disorders in children due to Pb exposure, are very common. According to the US Centers for disease control and prevention (CDC), 10 μg/dl or higher concentration of Pb in blood is considered as “elevated” or a “level of concern.” However, it has been shown that even blood Pb concentrations below 10 μg/dl are inversely associated with children's intelligence quotient (IQ) scores at three to five years of age. As a result of these findings, the CDC has recommended changes for the threshold of acceptable elevated blood Pb concentrations to 5 μg/dl.
6.3.2 Role of EDXRF in Diagnosis of Blood Lead Level
Lead (Pb) enters the body primarily by absorption through the gastrointestinal tract or by inhalation. It is estimated that about 11% of Pb in adults and 25–48% in children has been absorbed from the gastrointestinal track. After entering the body it infiltrates different bodily organs unequally. Pb concentrations in bone tissue rises from 8% to 90% after just 20 hours after entering in the body. Blood is the media responsible for transporting Pb throughout the body, and hence Pb can be monitored by measuring Pb concentrations in blood serum (S─Pb), blood plasma (P─Pb) or whole blood (W─Pb) samples. However, blood serum and blood plasma do not give a full indication of blood‐Pb level in a human body; therefore measurement Pb levels in whole blood is preferable to S─Pb or P─Pb measurements. According to Donald Smith et al. [14], Plasma Pb levels are approximately 0.29% on average of whole‐ blood Pb level.
Atomic absorption spectrometry (AAS), inductively coupled plasma‐atomic emission spectroscopy (ICP‐AES), polarography, and inductively coupled plasma mass spectroscopy (ICP‐MS) have been used for determining blood Pb levels in S─Pb or P─Pb for years. But this poses problems, namely that all these techniques require large volumes of samples. Moreover they cannot measure whole blood samples, as these contain fat bodies. However, collection of biological samples in large volumes is very complicated. On the other hand sample preparation for those instruments requires special attention and is thus time‐consuming. In this case EDXRF is the only suitable tool for measuring Pb concentrations in whole blood samples as it requires only small amounts of sample material, and is nondestructive and versatile in comparison with other techniques.
6.3.3 Collection of Blood Sample and Preparation
Five milliliters of venous whole blood was drawn by disposable syringe. The blood sample was transferred immediately into a labeled container and stored in an ultra‐freezer at −20 °C to avoid any microbial growth. Blood samples should be collected in plastic containers prior to pretreatment and kept in a 20% nitric acid solution for 24 hours, properly rinsed with deionized water, and dried. The empty weight of all containers were taken before being stored in a plastic covering. The total weight of blood with containers was taken at room temperature. Subtracting the empty weight of the container gave the weight of the fresh sample. The samples were dried in an oven at 60–70 °C for four to six days. The dried samples were powdered in a carbide mortar with the help of a pestle and preserved in desiccators until subsequent analysis [15].
6.3.4 Preparation of Pellets from Powdered Sample
For the preparation of pellets, 100 mg of powdered blood sample was pressed into a pellet having diameter of 1 cm and thickness of 1 mm using a pellet maker (CARVER, 10 mm, model no:018735C) applying 3 tons of pressure ( Figure 6.4). The pellet was then irradiated using an EDXRF machine [ Figure 6.5].
6.3.5 Sample Irradiation
Irradiation of the sample pellet was done with a 30 m Ci Cd‐109 radioisotope annular source for about one thousand seconds to excite the characteristic X‐rays of the elements present in the sample. The X‐rays were detected with the Si (Li) detector of 170 eV resolution. The X‐ray spectrum of each sample was collected by a multichannel analyzer and transferred to a computer for storage, processing, and evaluation of the net X‐ray intensities. Commercial software “AXIL” installed on the system was employed for the qualitative and quantitative analysis of the respective elements in the sample. Detailed of the machine set up and its working principles are described elsewhere [16, 17].
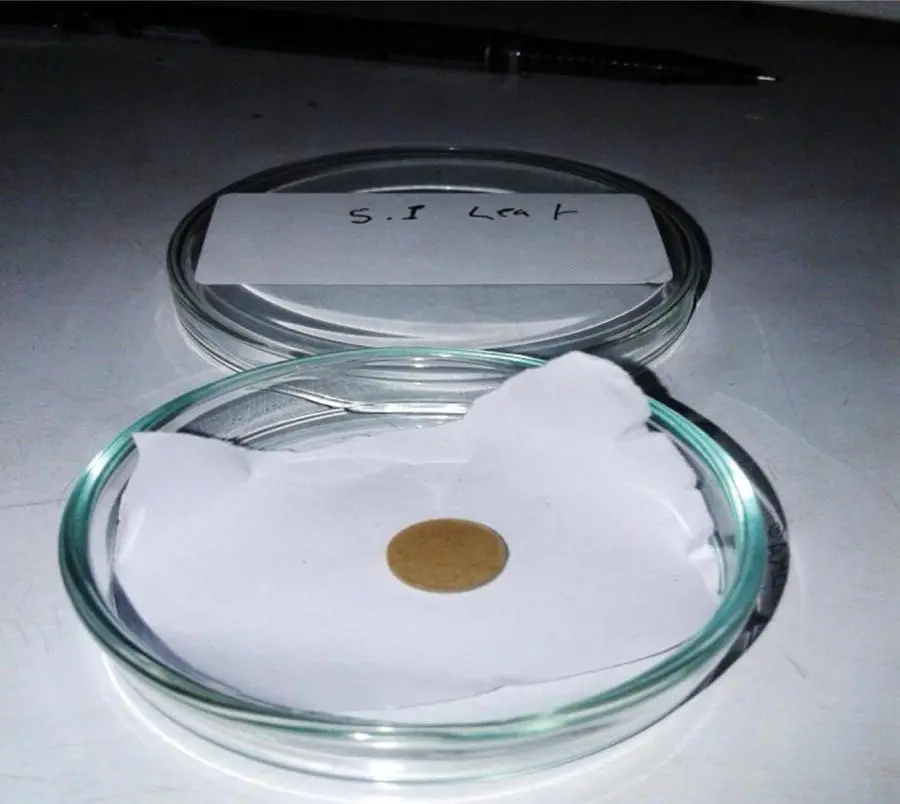
Figure 6.4 Pellet of blood sample.

Figure 6.5 Energy dispersive X‐ray fluorescence (EDXRF) system.
Читать дальшеИнтервал:
Закладка:
Похожие книги на «X-Ray Fluorescence in Biological Sciences»
Представляем Вашему вниманию похожие книги на «X-Ray Fluorescence in Biological Sciences» списком для выбора. Мы отобрали схожую по названию и смыслу литературу в надежде предоставить читателям больше вариантов отыскать новые, интересные, ещё непрочитанные произведения.
Обсуждение, отзывы о книге «X-Ray Fluorescence in Biological Sciences» и просто собственные мнения читателей. Оставьте ваши комментарии, напишите, что Вы думаете о произведении, его смысле или главных героях. Укажите что конкретно понравилось, а что нет, и почему Вы так считаете.