Increasing the receive coil Q with resistors is a poor strategy from the signal-to-noise ratio point of view since this introduces losses and thus radiofrequency noise. Lossless methods are needed to alter the frequency response of the tuned circuit. The Fano–Bode BW theory describes a general limit for the BW over which the impedance match of an MR coil can be achieved [135]. Cryoprobes at high field experience a similar issue [136]. In addition to traditional impedance matching methods, lossless manipulation of the tuned circuit frequency response can be addressed using a variant of preamplifier decoupling, typically used to reduce geometric coupling in arrays [137]. Traditional preamplifier decoupling uses a non-power-matched input impedance preamplifier to couple an extra parallel LC “trap” circuit to coil resonance. The lack of a power match is immaterial if the noise performance of the preamplifier is satisfactory with that matching condition. Because a power match is not used, it’s not clear if the Fano–Bode theory applies. Although known for causing a large perturbation to the coil’s frequency response, preamp decoupling at modest levels can create a minor split-resonance, which has the effect of lossless broadening the response. Figure 3.8 shows the frequency response of a low-impedance preamplifier (WanTcom Inc., Chanhassen, MN) connected to a coil tuned to 3.4 MHz. The strategy is able to reduce the coil’s power response variation over the desired imaging BW (shaded region of Figure 3.8) from 4.7 dB to 0.7 dB.
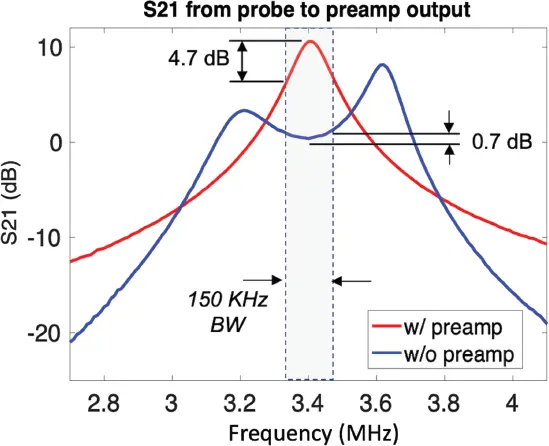
Figure 3.8 Response of a low-field magnetic resonance imaging coil with and without preamplifier decoupling. With mild preamplifier decoupling, the variation over the expected imaging bandwidth dropped from 4.7 dB to 0.7 dB.
3.5.2.3 External EMI Removal (Eliminating the Shielded Room)
Typical MRI scanners are sited inside a passively shielded conductive room (Faraday cage) to prevent external radiofrequency sources from inducing image artifacts. This >US$100 000 siting component is both costly and prevents portability. Passive shielding can be partially achieved by placing copper foil around the magnet. The passive radiofrequency shielding is generally sufficient for phantoms and objects that do not extend outside of the magnet (shielded area) but proves insufficient when a human subject is in the magnet. This is because the conductive human torso and legs act as an antenna which picks up external radiofrequency interference and pipes it into the detection coil. Imaging a cantaloupe and a living human can differ in external interference artifact by as much as 100-fold.
Additional EMI mitigation can be achieved using external interference detectors (additional radiofrequency coils outside of the magnet) and retrospective removal from the images. The interference is picked up from external coils and the artifact is removed from the image using a trained correlation model between that seen in imaging coil and the external detection coil. This approach has a broad history in electromagnetic measurements and in EEG and magnetoencephalography (MEG). Muller-Petke provides some review as well as an application to MR [138], and several methods have emerged specifically for low-field MRI systems [139,140]. Figure 3.9 shows results of an example phantom experiment in an 80-mT portable brain scanner [20,141] using retrospective EMI correction with five external detector coils [140]. Although refinements will continue, it appears that the EMI issue can be managed for POC MRI without a Faraday-shielded room.
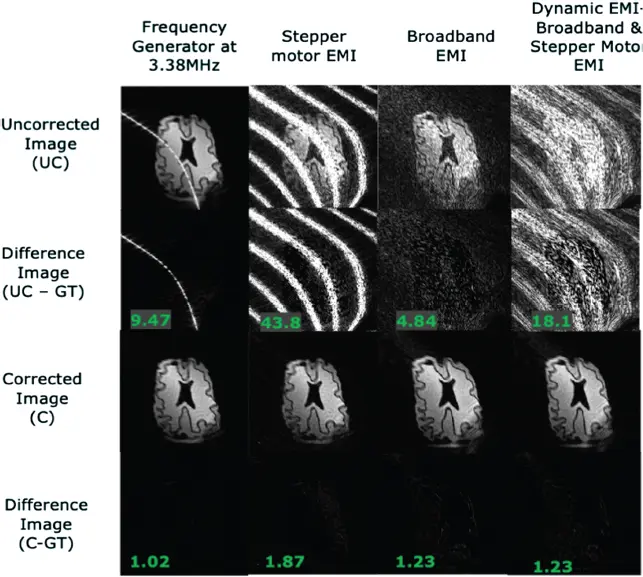
Figure 3.9 Retrospective EMI correction of a time-varying EMI using five external coils using a k-space correlation matrix approach in a low-field scanner [140]. Four electromagnetic interference (EMI) sources are shown including a dynamic source (right-hand column). The bottom row shows difference images using “Ground Truth” (GT) images obtained without the noise sources present.
Research in MRI technology has done a phenomenal job of expanding diagnostic benefit by developing acquisition techniques and instrumentation to enable MRI scanners to “see more” through improved sensitivity, spatio-temporal resolution, contrasts, and expanded clinical targets. In contrast to the clear benefits achieved in this direction, extending the reach of MRI by widening the range of where it can be applied has received less attention (until recently). This is especially seen for MRI technology designed to extend its use at the POC such as emergency medicine settings by reducing its cost, and siting needs such as footprint, power, cooling, venting, and shielding to the point where bedside use is possible. Despite the challenges, recent activity has advanced the technology on multiple fronts, making MRI scanners available in a wider range of settings; look for a portable MRI scanner if you are unfortunate enough to need a visit to the ED!
The authors would like to thank Jason Stockmann, Patrick McDaniel, Thomas Witzel, Mathew Rosen, and Abitha Srivnivas for their extensive technical help, and John Conklin, Sarah Bates, Farrah Mateen, and Michael Lev for their input to the clinical issues surrounding portable and POC MRI. We thank Alex Barksdale for the superconducting magnet optimizations. Research reported in this publication was supported by the National Institute of Biomedical Imaging and Bioengineering of the National Institutes of Health under award number R01EB018976.
1 1Buller, M.and Karis, J.P. (2017). Introduction of a dedicated emergency department MR imaging scanner at the Barrow Neurological Institute. American Journal of Neuroradiology 38 (8): 1480–1485.
2 2 Redd, V., Levin, S., Toerper, M.et al. (2015). Effects of fully accessible magnetic resonance imaging in the emergency department. Academic Emergency Medicine 22 (6): 741–749.
3 3Sanchez, Y., Yun, B.J., Prabhakar, A.M.et al. (2017). Magnetic resonance imaging utilization in an emergency department observation unit. The Western Journal of Emergency Medicine 18 (5): 780–784.
4 4Manna, S., Voutsinas, N., Maron, S.Z.et al. (2020). Leveraging IR’s adaptability during COVID-19: A multicenter single urban health system experience. Journal of Vascular and Interventional Radiology 31 (7): 1192–1194.
5 5Jacobi, A., Chung, M., Bernheim, A.et al. (2020). Portable chest X-ray in coronavirus disease-19 (COVID-19): A pictorial review. Clinical Imaging 64: 35–42.
6 6Chung, M., Bernheim, A., Mei, X.et al. (2020). CT imaging features of 2019 novel coronavirus (2019-nCoV). Radiology 295 (1): 202–207.
7 7El Homsi, M., Chung, M., Bernheim, A.et al. (2020). Review of chest CT manifestations of COVID-19 infection. European Journal of Radiology Open 7: 100239.
8 8Smith, M.J., Hayward, S.A., Innes, S.M.et al. (2020). Point-of-care lung ultrasound in patients with COVID-19 – A narrative review. Anaesthesia 75: 1096–1104.
9 9Kandemirli, S.G., Dogan, L., Sarikaya, Z.T.et al. (2020). Brain MRI findings in patients in the intensive care unit with COVID-19 infection. Radiology 297: 201697.
10 10Ogbole, G.I., Adeyomoye, A.O., Badu-Peprah, A.et al. (2018). Survey of magnetic resonance imaging availability in West Africa. The Pan African Medical Journal 30: 240.
Читать дальше








