1 ...7 8 9 11 12 13 ...29 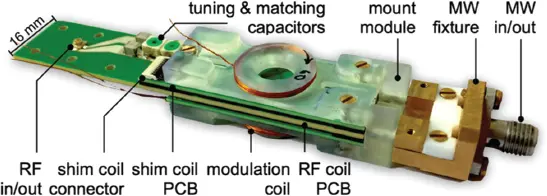
Figure 1.13 Photograph of a fully integrated Overhauser dynamic nuclear polarization (ODNP) probe head, designed to operate in a palm-held 0.5-T permanent magnet. [72] Sebastian Kiss (2019)/figure 05.26 [p.125]/with permission from University of Freiburg.
MR microscopy can benefit greatly from engineering approaches to bridge the gap between the sample and the spectrometer, especially in the application areas of cell and small organism biology, and (electro) chemistry. In this chapter we considered the contribution that microengineering can make, covering custom resonators and sample holders, which provide more ideal SNR and sample conditions, thus facilitating increased experimental flexibility. In essence, Faraday induction NMR detection has arrived at the LOD, so research work is currently mainly focusing on system issues, dealing with complex samples, achieving better field shimming, hyphenation of measurement modalities, and hyperpolarization of the spin population, and of course achieving spectrometer agility and proximity. Certainly, quantum sensors may provide more sensitivity in the future, but also require proximity of the detection system to the spin of interest, which for many applications is tantamount to becoming invasive. It is also not yet clear how hyperpolarization can be made less invasive, or less poisonous. The future will see small detectors becoming easier to use, not least because of better integration into the NMR workflow and equipment. Already some micro-NMR and hyperpolarization startups are pushing the field in this direction.
1 1Callaghan, P.T. (1991). Principles of Nuclear Magnetic Resonance Microscopy. Oxford, UK: Clarendon Press.
2 2Mansfield, P.and Grannell, P.K. (1973). NMR “diffraction” in solids. Journal of Physics C 6: L422–L426.
3 3Blümler, P., Greferath, G., Blümich, B.et al. (1993). NMR imaging of objects containing similar substructures. Journal of Magnetic Resonance A 103: 142–150.
4 4Blümich, B. (2016). k and q dedicated to Paul Callaghan. Journal of Magnetic Resonance A 267: 79–85.
5 5Marx, V. (2013). Is super-resolution microscopy right for you? Nature Methods 10: 1157–1163.
6 6Ciobanu, L., Seeber, D.A., and Pennington, C.H. (2002). 3D MR microscopy with resolution 3.7 µm by 3.3 µm by 3.3 µm. Journal of Magnetic Resonance 158 (1–2): 178–182.
7 7Rugar, D., Budakian, R., Mamin, H.et al. (2004). Single spin detection by magnetic resonance force microscopy. Nature 430: 329–332.
8 8Bar-Even, A., Noor, E., Savir, Y.et al. (2011). The moderately efficient enzyme: Evolutionary and physicochemical trends shaping enzyme parameters. Biochemistry 50 (21): 4402–4410.
9 9Chen, H.-Y., Aggarwal, R., Bok, R.A.et al. (2020). Hyperpolarized 13C-pyruvate MRI detects real-time metabolic flux in prostate cancer metastases to bone and liver: A clinical feasibility study. Prostate Cancer and Prostatic Diseases 23 (2): 269–276. doi: 10.1038/s41391-019-0180-z.
10 10Staudacher, T., Shi, F., Pezzagna, S.et al. (2013). Nuclear magnetic resonance spectroscopy on a (5-nanometer)3 sample volume. Science 339 (6119): 561–563. doi: 10.1126/science.1231675.
11 11Cho, Z.H., Ahn, C.B., Juh, S.C.et al. (1988). Nuclear magnetic resonance microscopy with 4-µm resolution: Theoretical study and experimental results. Medical Physics 15 (6): 815–824. doi: 10.1118/1.596287.
12 12 Ahn, C.B.and Cho, Z.H. (1989). A generalized formulation of diffusion effects in µm resolution nuclear magnetic resonance imaging. Medical Physics 16 (1): 22–28. doi: 10.1118/1.596393.
13 13 McFarland, E.W.and Mortara, A. (1992). Three-dimensional NMR microscopy: Improving SNR with temperature and microcoils. Magnetic Resonance Imaging 10 (2): 279–288. doi: 10.1016/0730-725X(92)90487-K.
14 14Brandl, M.and Haase, A. (1994). Molecular diffusion in NMR microscopy. Journal of Magnetic Resonance. Series B 103 (2): 162–167.
15 15Ahn, C.B.and Chu, W.C. (1969). Optimal imaging strategies for three-dimensional nuclear magnetic resonance microscopy. Journal of Magnetic Resonance 94 (3): 455–470. doi: 10.1016/0022-2364(91)90132-D.
16 16Blackband, S.J., Buckley, D.L., Bui, J.D.et al. (1999). NMR microscopy – Beginnings and new directions. Magnetic Resonance Materials in Physics, Biology and Medicine 9 (3): 112–116.
17 17Mansfield, P. (1982). NMR Imaging in Biomedicine: Supplement 2 Advances in Magnetic Resonance, Volume 2. Amsterdam: Elsevier.
18 18Korvink, J.G., MacKinnon, N., Badilita, V.et al. (2019). “Small is beautiful” in NMR. Journal of Magnetic Resonance 306: 112–117. doi: 10.1016/j.jmr.2019.07.012.
19 19Webb, A.G. (2013). Radiofrequency microcoils for magnetic resonance imaging and spectroscopy. Journal of Magnetic Resonance 229: 55–66. doi: 10.1016/j.jmr.2012.10.004.
20 20 Fratila, R.M.and Velders, A.H. (2011). Small-volume nuclear magnetic resonance spectroscopy. Sensors and Actuators A: Physical 4 (1): 227–249. doi: 10.1146/annurev-anchem-061010-114024.
21 21 Kentgens, A.P.M., Bart, J., Van Bentum, P.J.M.et al. (2008). High-resolution liquid-and solid-state nuclear magnetic resonance of nanoliter sample volumes using microcoil detectors. Journal of Chemical Physics 128 (5): 052202. doi: 10.1063/1.2833560.
22 22Badilita, V., Meier, R.C., Spengler, N.et al. (2012). Microscale nuclear magnetic resonance: A tool for soft matter research. Soft Matter 8 (41): 10583–10597.
23 23Ciobanu, L., Seeber, D.A., and Pennington, C.H. (2002). 3D MR microscopy with resolution 3.7 μm by 3.3 μm by 3.3 μm. Journal of Magnetic Resonance 158 (1–2): 178–182. doi: 10.1016/S1090-7807(02)00071-X.
24 24 Gruschke, O.G., Baxan, N., Clad, L.et al. (2012). Lab on a chip phased-array MR multi-platform analysis system. Lab on a Chip 12 (3): 495–502. doi: 10.1039/c2lc20585h.
25 25Roffmann, W.U., Crozier, S., Luescher, K.et al. (1996). Small birdcage resonators for high-field NMR microscopy. Journal of Magnetic Resonance. Series B 111 (2): 174–177.
26 26Spengler, N., Moazenzadeh, A., Meier, R.et al. (2014). Micro-fabricated Helmholtz coil featuring disposable microfluidic sample inserts for applications in nuclear magnetic resonance. Journal of Micromechanics and Microengineering 24 (3): 034004. doi: 10.1088/0960-1317/24/3/034004.
27 27Schnall, M.D., Barlow, C., Subramanian, V.H.et al. (1969). Wireless implanted magnetic resonance probes for in vivo NMR. Journal of Magnetic Resonance 68 (1): 161–167. doi: 10.1016/0022-2364(86)90326-4.
28 28 Wirth, E.D., III, Mareci, T.H., Beck, B.L.et al. (1993). A comparison of an inductively coupled implanted coil with optimized surface coils for in vivo NMR imaging of the spinal cord. Magnetic Resonance in Medicine 30 (5): 626–633. doi: 10.1002/mrm.1910300514.
29 29 Silver, X., Ni, W.X., Mercer, E.V.et al. (2001). In vivo 1H magnetic resonance imaging and spectroscopy of the rat spinal cord using an inductively-coupled chronically implanted RF coil. Magnetic Resonance in Medicine 46 (6): 1216–1222. doi: 10.1002/mrm.1319.
30 30 Elshafiey, I., Bilgen, M., He, R.et al. (2002). In vivo diffusion tensor imaging of rat spinal cord at 7 T. Magnetic Resonance Imaging 20 (3): 243–247. doi: 10.1016/S0730-725X(02)00493-9.
31 31 Quick, H.H., Kuehl, H., Kaiser, G.et al. (2002). Inductively coupled stent antennas in MRI. Magnetic Resonance in Medicine 48 (5): 781–790. doi: 10.1002/mrm.10269.
32 32 Ginefri, J.-C., Rubin, A., Tatoulian, M.et al. (2012). Implanted, inductively-coupled, radiofrequency coils fabricated on flexible polymeric material: Application to in vivo rat brain MRI at 7T. Journal of Magnetic Resonance 224: 61–70. doi: 10.1016/j.jmr.2012.09.003.
Читать дальше







