4.2.2. Transition‐Metal/Chiral Cation Dual Catalysis
Asymmetric transition‐metal catalysis has traditionally relied on the use of chiral ancillary ligands to modulate the metal’s primary coordination sphere to directly influence enantioselectivity. In recent years, a distinct strategy has emerged wherein ion‐pairing interactions at a metal’s secondary coordination sphere can influence enantioselectivity. Given that cationic metal complexes are more common than their anionic counterparts in catalysis, implementation of this strategy using chiral‐anions has seen more progress and will be discussed in detail in Section 3.6. Conversely, ion‐pairing between a chiral cation and an anionic metal complex has also been reported. In 2016, Tan reported the use of a dinuclear peroxomolybdate anionic oxidant ion‐paired to a chiral bisguanidinium dication catalyst for the enantioselective oxidation of various sulfides to the corresponding sulfoxides ( Scheme 4.25) [81]. This proposed catalytically relevant ion‐pair was isolable and characterized by single‐crystal X‐ray diffraction. The same year, a related system was reported by the same group using a tungstate catalyst and the identical chiral bisguanidinium catalyst [82]. Prior to this work, chiral cationic catalysts had been reported to ion‐pair with anionic metal complexes such as permanganate anions when used stoichiometrically [83, 84].
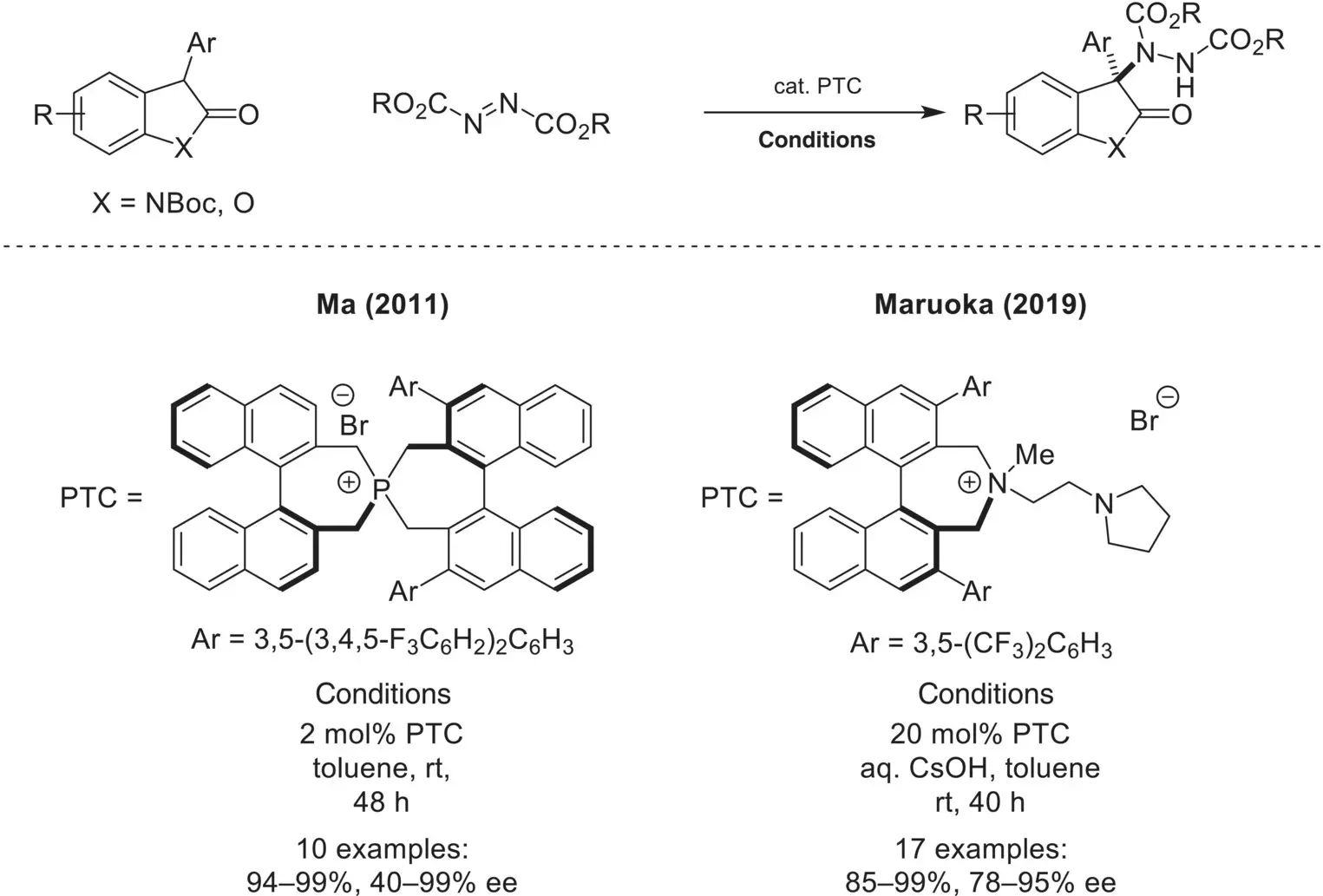
Scheme 4.23. Enantioselective α‐amination of oxindoles and lactones using azodicarboxylates.
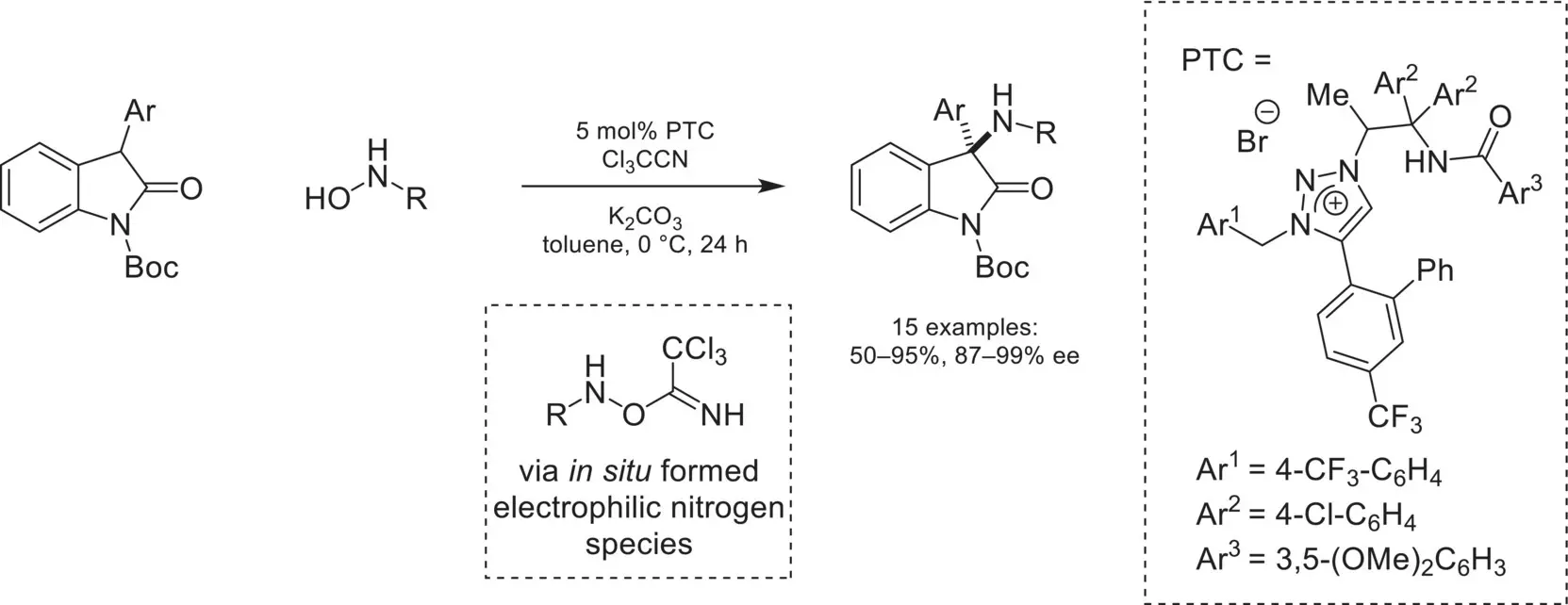
Scheme 4.24. Enantioselective α‐amination of oxindoles using hydroxylamines.
Source: Based on [80].

Scheme 4.25. Enantioselective oxidation of sulfides using a peroxomolybdate/chiral cation ion pair catalyst.
Source: Based on [81].
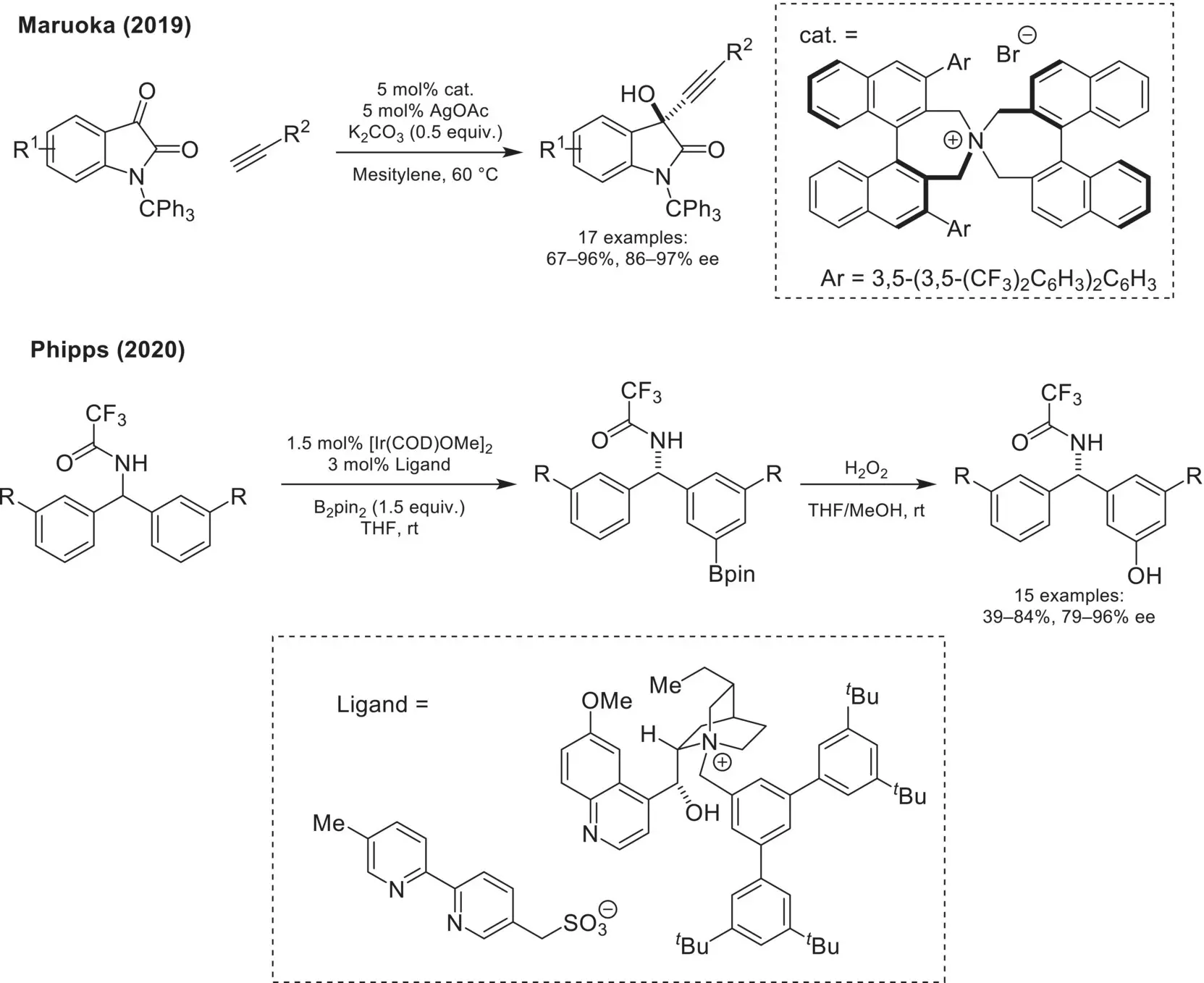
Scheme 4.26. Enantioselective transformations via transition metal/chiral cation ion pairing catalysis.
The asymmetric addition of alkynyl nucleophiles to carbonyl‐containing compounds represents a powerful approach to form chiral propargyl alcohols. In 2019, Maruoka demonstrated that a chiral ammonium catalyst could ion‐pair with a catalytically generated Ag‐alkynylide for the enantioselective addition to isatin derivatives ( Scheme 4.26) [85]. While chiral ion‐pairing and phase‐transfer catalyses have typically involved ion‐pairing with an enolate nucleophile, this represents a unique example where an alternative carbon‐based nucleophile can be productively used via the use of a transition‐metal co‐catalyst. In 2020, the Phipps group disclosed an elegant system for the desymmetrization of geminal diaryl derivatives via enantioselective Ir‐catalyzed C–H borylation [86]. The key ion‐pairing between a strategically placed sulfonate group on the bipyridine ligand backbone and a chiral cinchoninium cation enables the enantioselective meta‐selective borylation of prochiral diaryl substrates. This work is particularly significant as it represents a rare example of remote asymmetric induction, where the site of the newly formed C–B bond is far from the newly formed chiral center. Looking forward, the strategy of incorporating chiral ion‐pairing interactions in ligand design for metal‐catalyzed transformations is positioned to have significant impact for the development of new reactions with unique selectivity profiles.
4.2.3. Cation‐Binding Catalysis
Since the discovery of the cation‐binding properties of cyclic polyethers, there has been a desire to utilize this class of compounds to impart enantioselectivity onto a reaction by utilizing a chiral crown ether. The first example of this concept was demonstrated by Cram in 1981, with the asymmetric Michael addition of a cyclic β‐keto ester into methyl vinyl ketone (MVK) with a BINOL‐derived 22‐crown‐6 catalyst ( Scheme 4.27) [87]. In this example, a chiral crown ether acts as a phase‐transfer catalyst for KO t Bu. After deprotonation of the substrate, a potassium‐bound crown ether cation is ion‐paired with an enolate, allowing for enantioselective addition to methyl vinyl ketone (MVK).
Since the initial report by Cram, focus in the field of cation‐binding catalysis shifted to utilizing crown ether catalysts derived from carbohydrates. In 1989, a highly symmetric polyether catalyst was reported that catalyzed an asymmetric Michael addition of methyl phenyl acetate to methyl acrylate in high yield and moderate enantioselectivity ( Scheme 4.28) [88]. In 1997, the Bakó group reported that an aza‐crown ether catalyst derived from D‐glucose catalyzed a nitro‐Michael reaction in high enantioselectivity [89]. This catalyst architecture proved to be applicable in various asymmetric phase‐transfer settings, such as in glycine imine[90] and aminomethylene phosphonate alkylation[91], as well as asymmetric chalcone epoxidation [92].
In 2009, the Song group demonstrated a novel polyether catalyst based on the BINOL scaffold that competently bound and phase‐transferred KF ( Scheme 4.29) [93]. This catalyst was effective for the kinetic resolution of silyl‐protected alcohols via desilylation [94]. A 2015 follow‐up publication revealed that this catalyst architecture was competent for the reverse‐reaction, where an alcohol was kinetically resolved by silylation with the catalyst [95]. In early 2016, the Yan group applied this system to kinetic resolution via an E1cB‐elimination of β‐sulfonyl ketones [96]. Over the next several years, numbers of other kinetic resolutions featuring various leaving groups were shown to be compatible with this strategy, such as poly halogenated ketones[97], and aldols [98].
In addition to initiation with KF, in 2012, an organocatalytic asymmetric Strecker reaction was developed utilizing BINOL‐derived crown ether with KCN ( Scheme 4.30) [99]. The functionalization of α‐amidosulfones proved to be a valuable paradigm, as many compatible anions were demonstrated to perform Mannich reactivity in high yields and selectivities. Notable nucleophiles include fluoro‐oxindoles [100], indoles [101], fluoroketones [102], thiocyanato ketones [103], and phthalimides [104]. In addition to kinetic resolutions, this system was also found to be useful for the synthesis of cyclic compounds from unsaturated ketones, such as with nitrones [105], mercaptoacetaldehyde [106], or intramolecular exo ‐trig cyclizations [107].
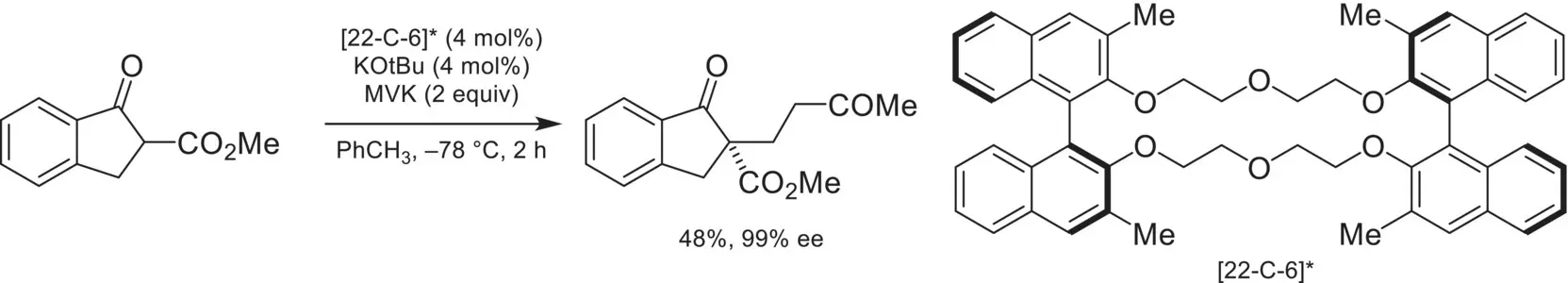
Scheme 4.27. Asymmetric Michael addition via cation binding BINOL‐derived ether catalysts.
Source: Based on [87].
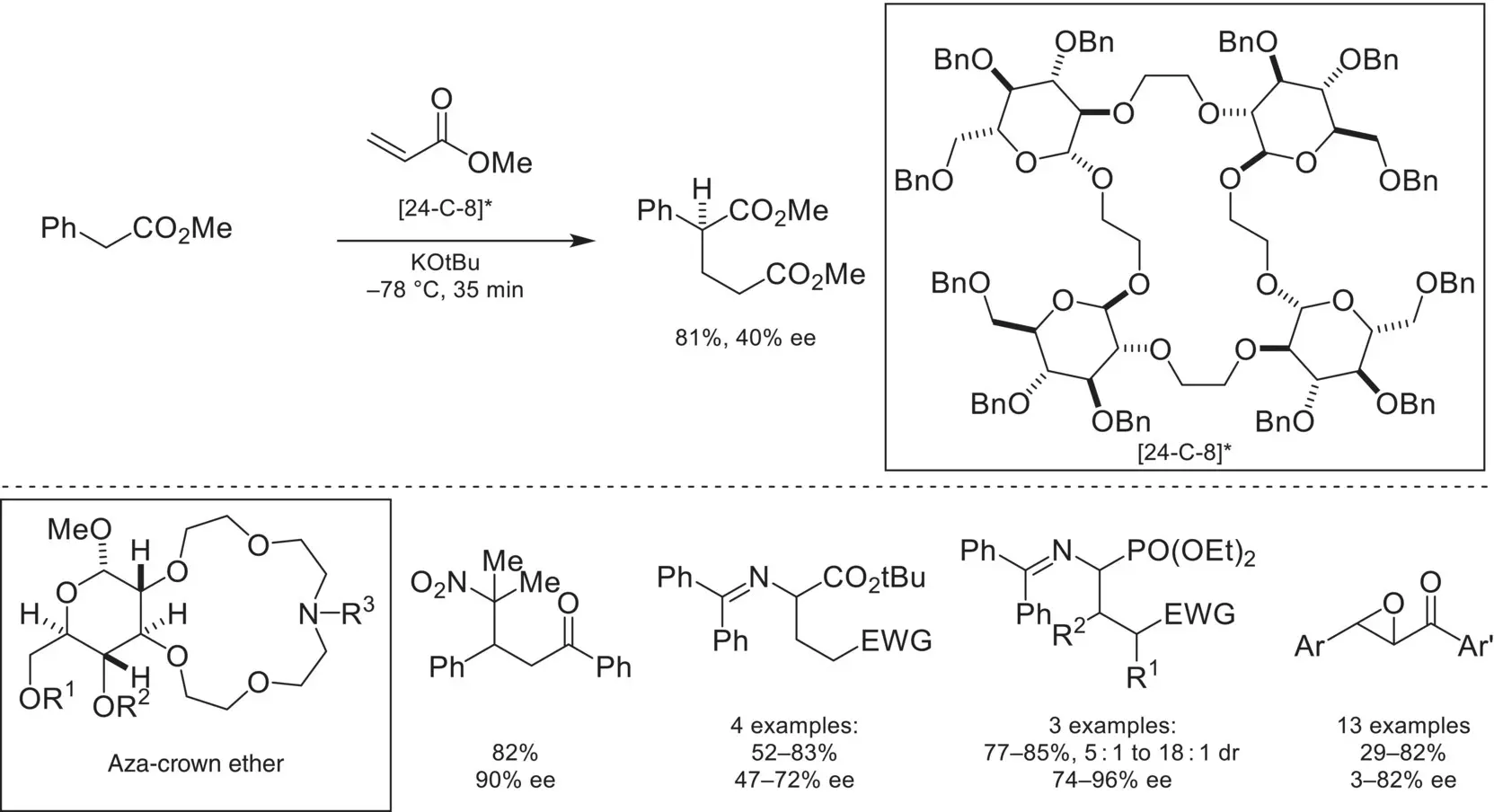
Scheme 4.28. Enantioselective Michael addition via cation binding sugar‐derived ether catalysts.
Читать дальше













