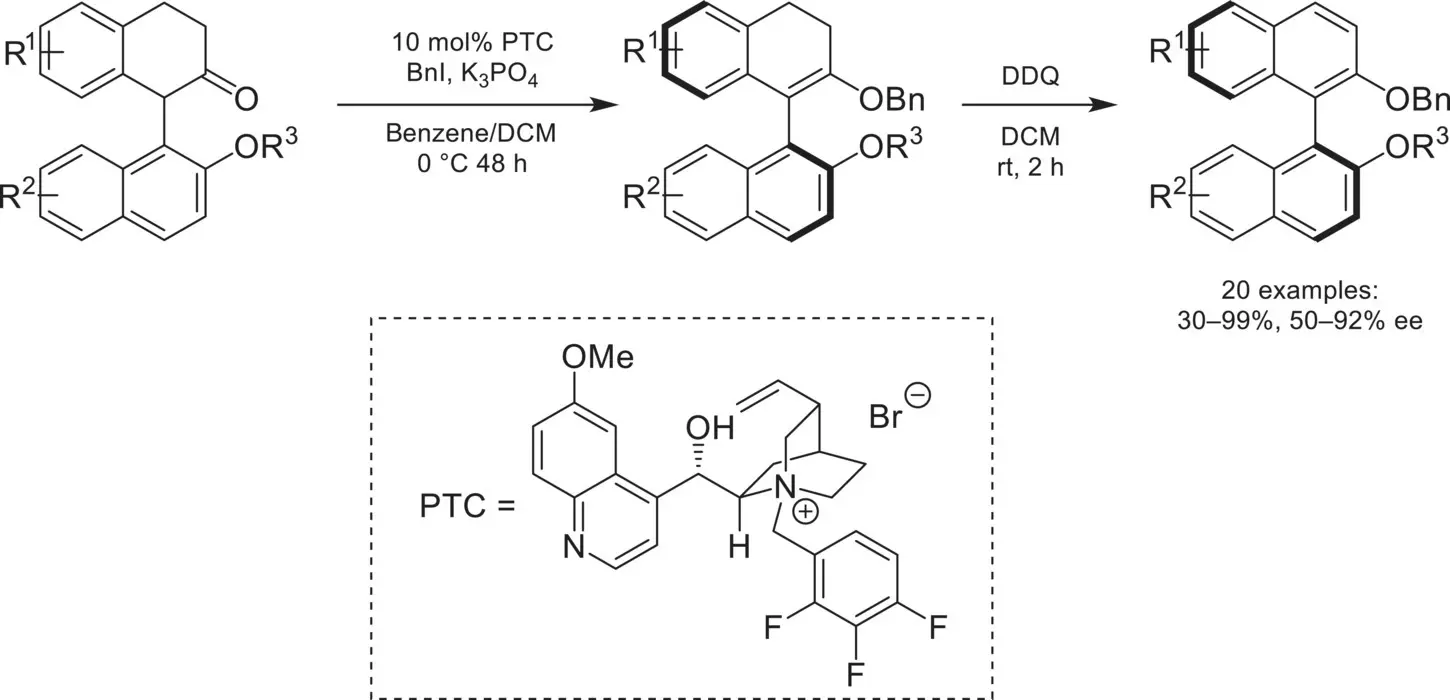
Scheme 4.6. Atropselective enolate O‐alkylation.
The asymmetric oxidation of α,β‐unsaturated carbonyl compounds using phase‐transfer catalysis was also extensively studied in the early years of the field as new chiral cationic catalysts were being developed. In 1998, Shioiri and Arai reported the epoxidation of chalcone using aqueous hydroperoxide as the oxidant and a chiral cinchoninium catalyst ( Scheme 4.8) [41, 42]. Later, Jew and Park reported that a tethered bis‐cinchoninium catalyst could catalyze the same reaction to provide the corresponding epoxide with excellent enantioselectivity [43]. Interestingly, the use and optimization of a surfactant (Span 20) improved both the rate of the reaction and the enantioselectivity, presumably due to increased surface area between the organic and aqueous phases or the formation of micelles. Finally, a chiral cationic aza‐crown ether catalyst was shown to promote the same reaction with moderate enantioselectivity [44]. A number of other groups also reported the phase‐transfer catalyzed epoxidation of chalcones and related compounds, using alternative oxidants such as hypochlorite salts [45–47].
A related phase‐transfer strategy was also used for the enantioselective aziridination of α,β‐unsaturated carbonyl compounds, using N‐acyl hydroxylamines as the “nitrene equivalent” source. Aires‐de‐Sousa first reported the aziridination of acrylate derivatives using first‐generation chiral cinchoninium catalysts ( Scheme 4.9) [48]. This reaction proceeds via the intramolecular rearrangement of the N‐acylhydroxylamine anion to an O‐acylhydroxylamine anion under basic conditions. Conjugate addition of the ion‐paired catalyst/N‐acylhydroxylamine to the acrylate electrophile is followed by intramolecular cyclization and liberation of pivalate. Later work by Murugan and Siva demonstrated the use of modified chiral cinchoninium catalysts for the highly enantioselective aziridination of acrylates [49]. Specifically, tosylation of the free alcohol in the ammonium catalyst provided high levels of enantioinduction.
Building on these studies using phase‐transfer catalysis to achieve 1,4‐conjugate additions with O‐ and N‐based nucleophiles, hydrazide derivatives were later shown to be suitable nucleophiles. In 2010, Brière demonstrated the use of a chiral cinchoninium catalyst for the enantioselective synthesis of pyrazolines ( Scheme 4.10) [50]. Ion‐pairing of a hydrazide anion with the chiral ammonium catalyst allowed the enantioselective aza‐Michael addition with chalcones, followed by cyclocondensation to afford the pyrazoline product.
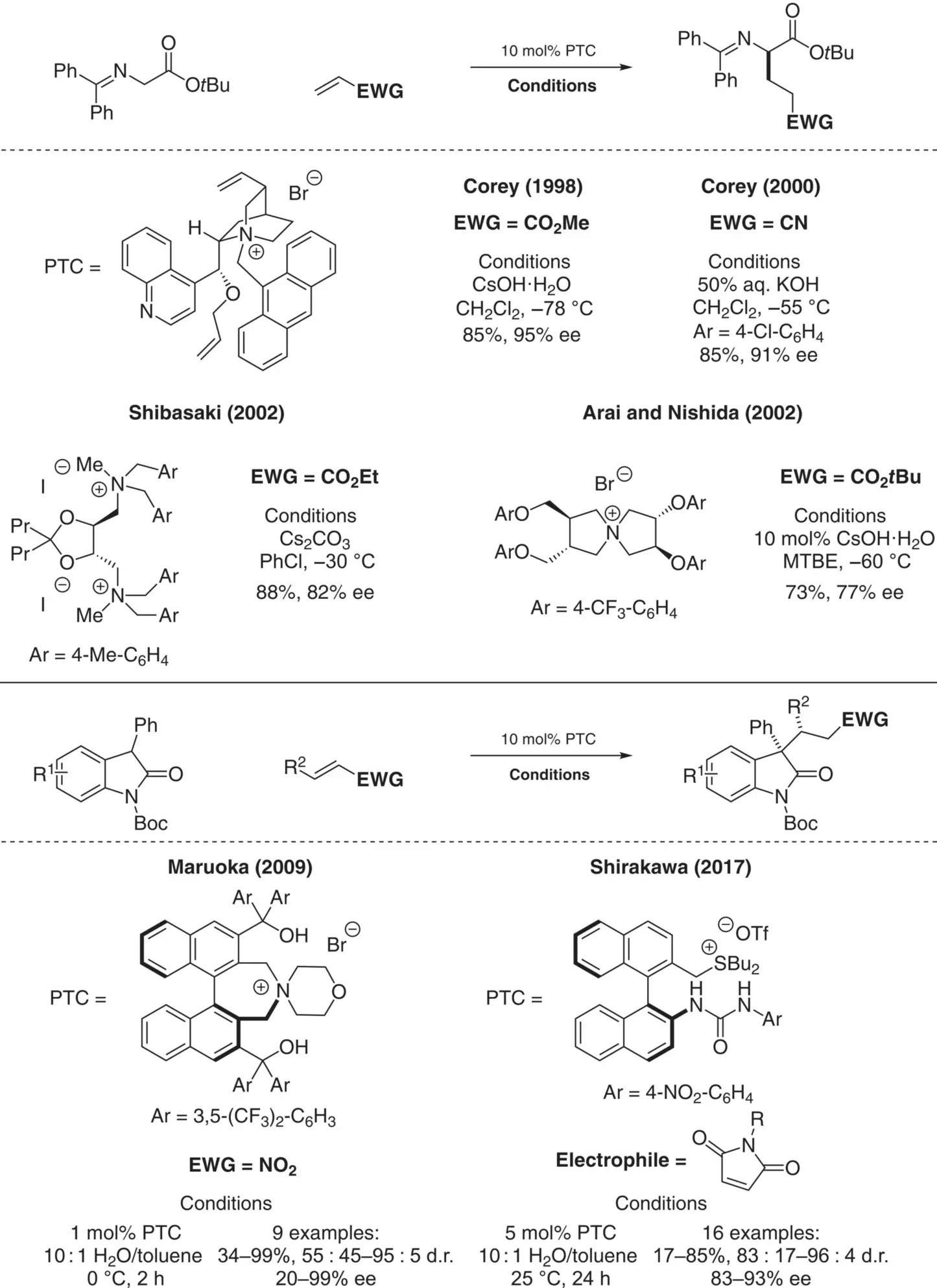
Scheme 4.7. Enantioselective addition of glycine imines to Michael acceptors.
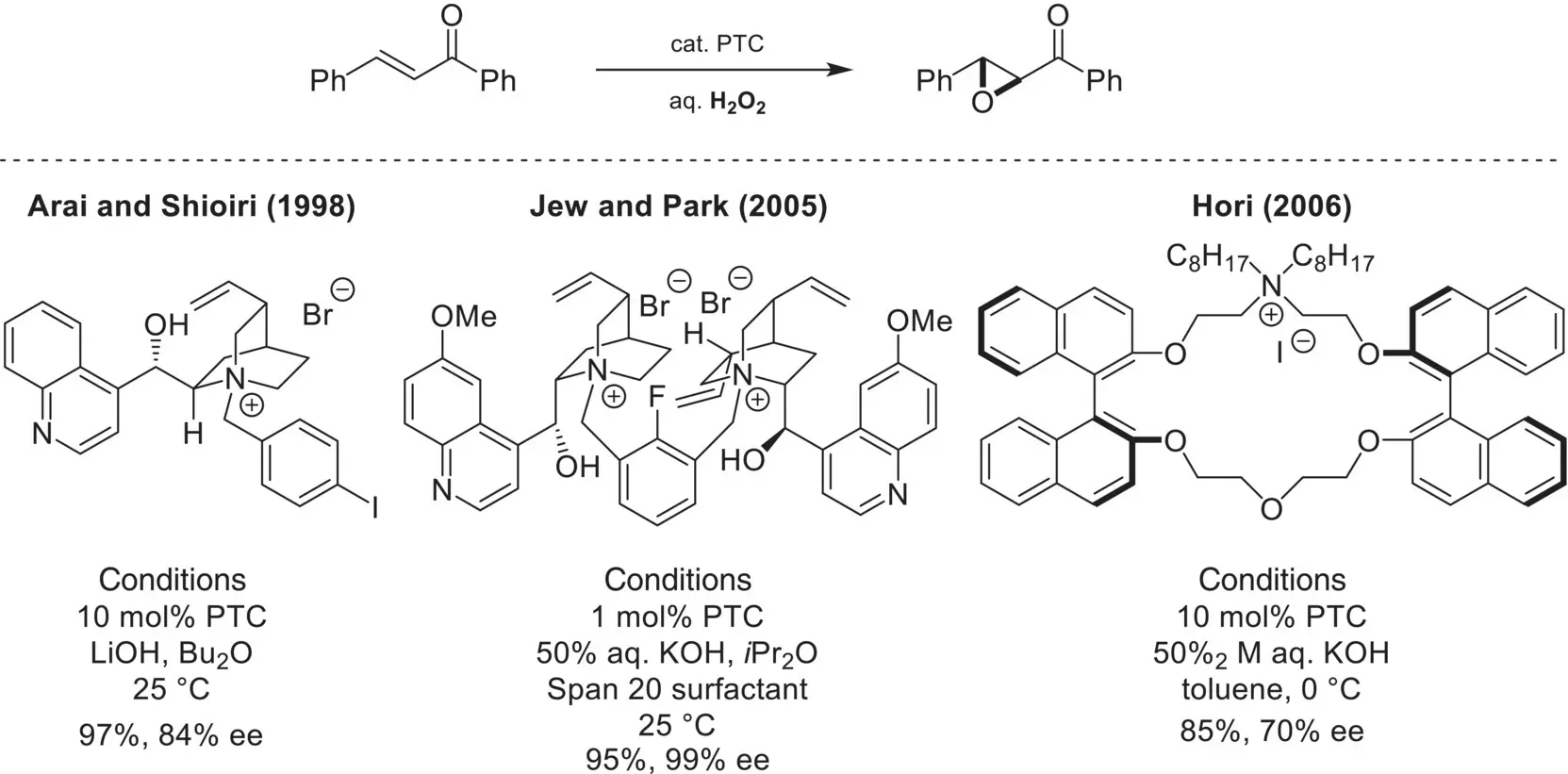
Scheme 4.8. Enantioselective epoxidation of chalcones.
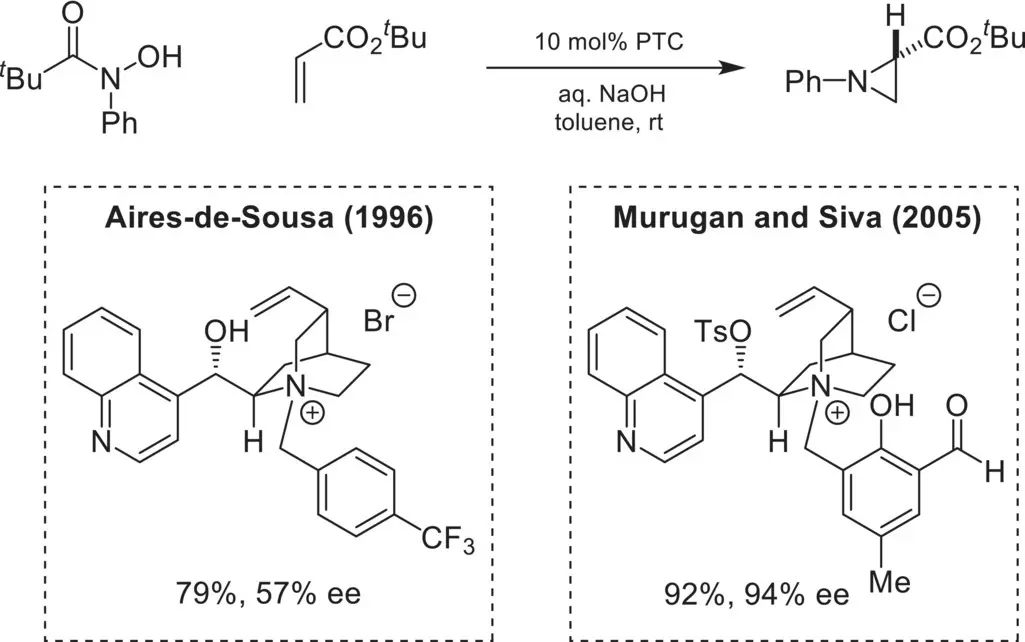
Scheme 4.9. Enantioselective aziridination of α,β‐unsaturated esters.
4.2.1.3. Addition to Carbonyls and Imines
Compared to alkylation reactions, phase‐transfer catalyzed aldol reactions have been comparatively underexplored. In 1991, Miller reported the aldol reaction of diphenyl glycine imine nucleophiles and various aldehydes with chiral cinchoninium‐based catalysts ( Scheme 4.11) [51]. Unfortunately, these reactions suffered from very low enantioselectivity (3–12% ee). Over the years, improvements using cinchoninium‐based catalysts have been reported for related aldol reactions, but still suffered from moderate enantioselectivity (up to 83% ee) [52]. In 2002, Maruoka reported a highly enantioselective phase‐transfer catalyzed aldol reaction using chiral N‐spiro ammonium catalysts (up to 98% ee) [53, 54]. Mechanistic studies revealed competitive retro‐aldol reactions were occurring under first‐generation conditions, but this was solved with the use of dilute and catalytic sodium hydroxide and ammonium chloride.
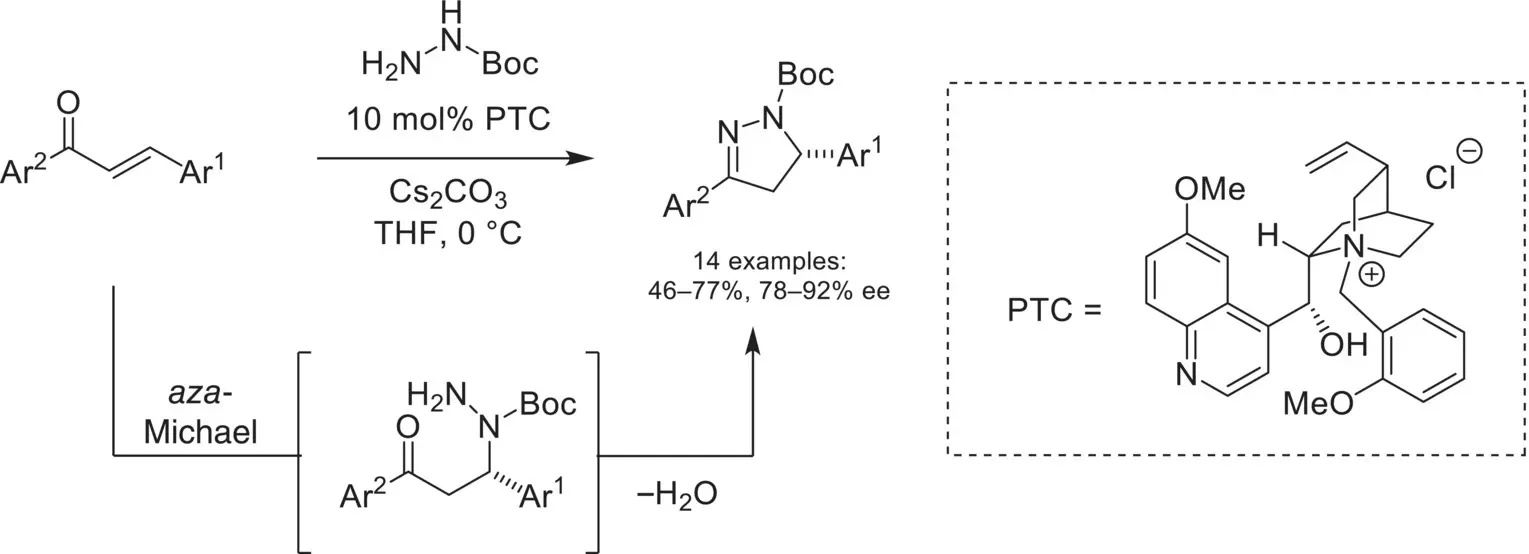
Scheme 4.10. Enantioselective aza‐Michael addition of hydrazides to chalcones for the synthesis of pyrazolines.

Scheme 4.11. Enantioselective aldol reaction using glycine imines.
Source: Based on [51].
The same family of chiral N‐spiro ammonium salts was later reported by Maruoka in 2004 as selective phase‐transfer catalysts for the related Mannich reaction of a diphenyl glycine ester with a N‐PMP protected α‐imino‐ester ( Scheme 4.12) [55]. A year later, Oshima and Shibasaki reported the use of a tartrate‐derived chiral bis‐ammonium catalyst as an efficient catalyst for the Mannich reactions using Boc‐protected aldimines [56].
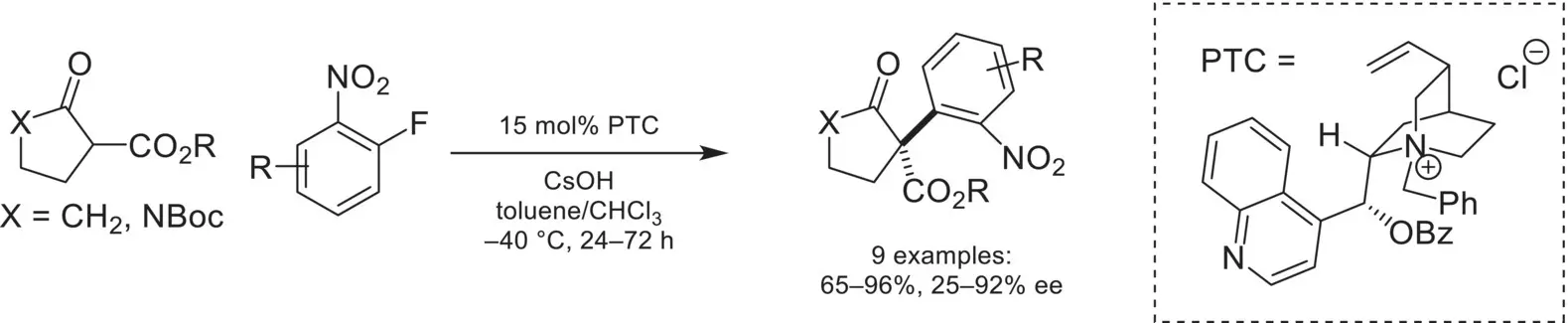
Asymmetric phase‐transfer catalysis has also been exploited successfully for a number of other reactions involving the addition of an enolate to a carbonyl or imine electrophile. For example, the addition of α‐halo carbonyl compounds to aldehydes or ketones (Darzens reaction) to yield epoxides was first achieved using chiral phase‐transfer catalysis in 1998 by Arai and Shioiri and has since been further developed [57, 58]. Another example is the addition of cyanide nucleophiles to imine derivatives (Strecker reaction) leading to chiral α‐amino nitriles, which has been extensively studied; the first highly enantioselective phase‐transfer catalyzed version of this reaction was reported by Maruoka in 2006 [59, 60].
In 2005, Jørgensen reported the first enantioselective S NAr arylation of enolates using chiral phase‐transfer catalysis ( Scheme 4.13) [61, 62]. β‐ketoesters could be arylated with activated aryl fluorides using a cinchoninium‐based catalyst. Notably, quaternary carbon centers were formed obviating the potential issue of product racemization.
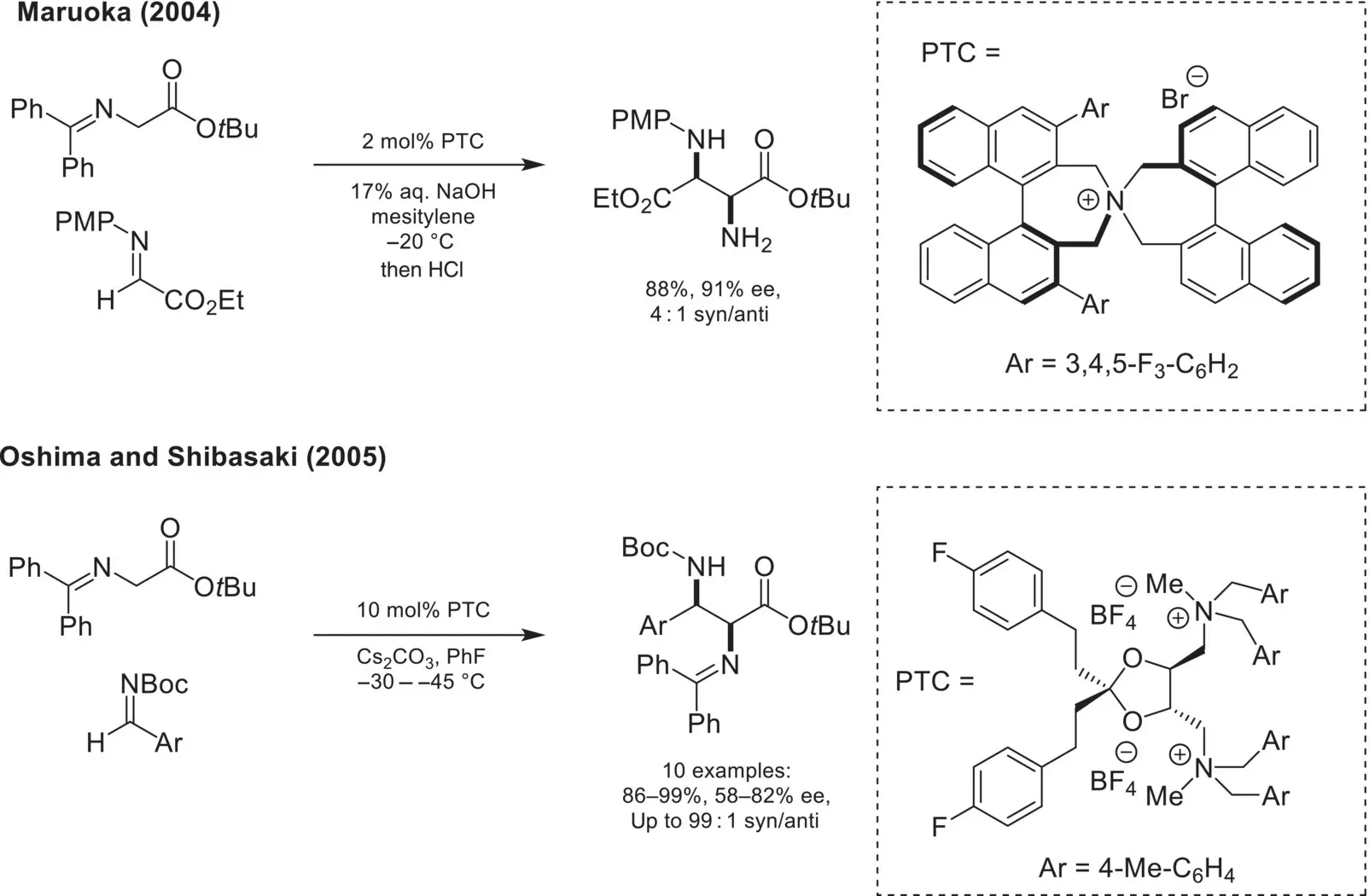
Scheme 4.12. Enantioselective Mannich reaction using glycine imines.
Scheme 4.13. Enantioselective arylation of 1,3‐dicarbonyl nucleophiles via nucleophilic aromatic substitution.
Source: [61, 62].
In 2014, Maruoka used a similar S NAr PTC strategy to achieve the enantioselective arylation of glycine imine derivatives ( Scheme 4.14) [63]. Here, a chiral ammonium catalyst with a biaryl backbone proved to be optimal. This chemistry was further expanded to use electron neutral and electron‐rich aryl electrophiles, activated as Cr(CO) 3complexes [64]. Use of a chiral binaphthyl‐derived ammonium catalyst proved to be optimal in this case.
Читать дальше















