In a further extension of this work, the arylation of oxindoles with activated aryl fluorides was accomplished using a binaphthyl‐derived chiral phosphonium catalyst ( Scheme 4.15) [65].
In recent years, the atropselective synthesis of biaryl motifs using chiral phase‐transfer catalysis has garnered increased attention. In 2014, Smith disclosed the first atropselective S NAr reaction using a dichloropyrimidine electrophile and a thiophenol nucleophile, wherein restricted rotation around the biaryl linkage in the product gives rise to atropisomers ( Scheme 4.16) [66]. Moderate to high enantioselectivity is observed using a first‐generation chiral cinchoninium catalyst. Building on this work using the same cinchoninium catalyst and related S NAr strategy, Gustafson later reported in 2018 the kinetic resolution of pyrolopyrimidines [67].
In 2017, Cai reported a chiral phase‐transfer catalyzed atropselective macrocyclization strategy to access (−)‐pterocarine and (−)‐galeon ( Scheme 4.17) [68]. A chiral cinchoninium catalyst was found to catalyze the intramolecular S NAr reaction of a phenol with an activated aryl fluoride to obtain the desired macrocyclic product with moderate to good enantioselectivity.
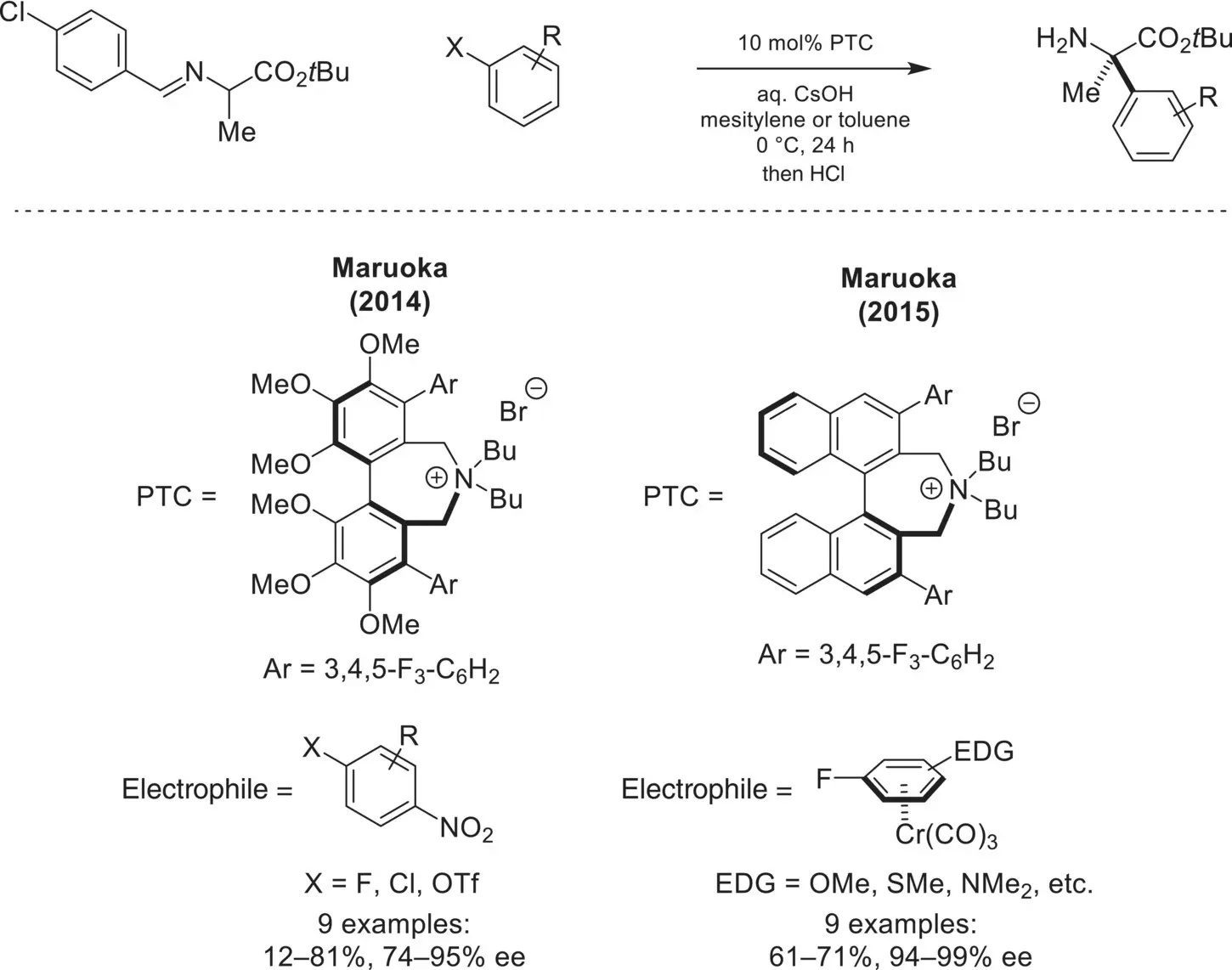
Scheme 4.14. Enantioselective arylation of mono‐alkylated glycine imines via nucleophilic aromatic substitution.
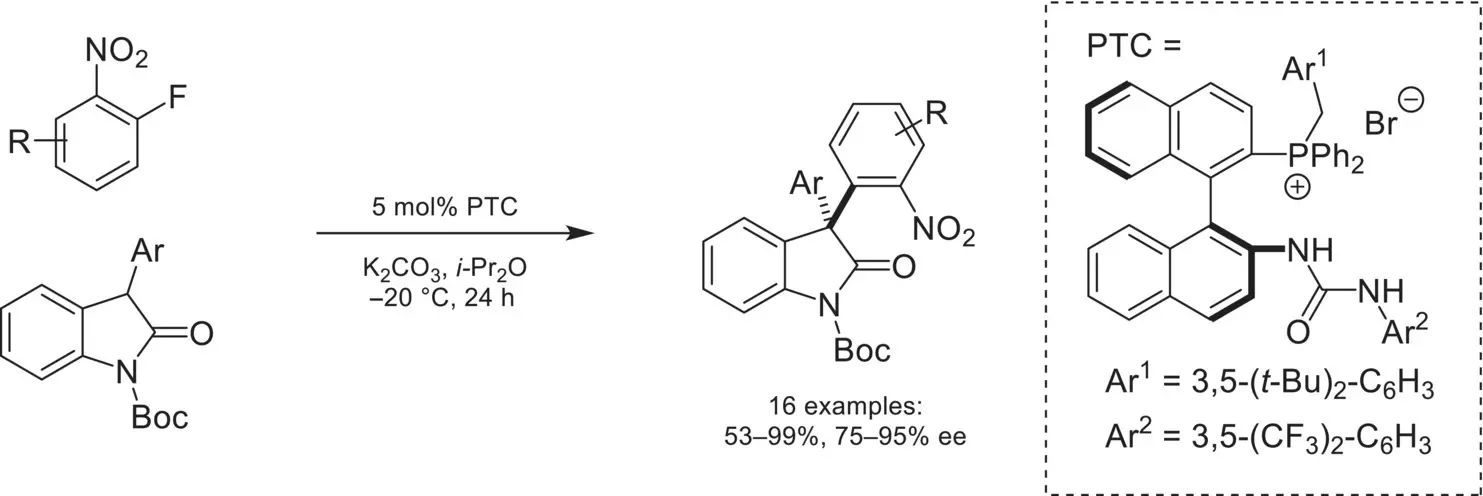
Scheme 4.15. Enantioselective arylation of oxindoles via nucleophilic aromatic substitution.
Source: Based on [65].
4.2.1.5. Carbon‐Heteroatom (C‐X) Bond Formation
The importance of enantioselective carbon‐heteroatom bond‐forming reactions cannot be overstated seeing as C–X (X = halogen, O, N, etc.) bonds are ubiquitous in pharmaceuticals and agrochemicals. Over the years, phase‐transfer catalysis has proven to be a privileged strategy to achieve these transformations. Among the most important factors for the successful implementation of such strategies is the development and use of suitable electrophilic heteroatom transfer reagents.
The strategic incorporation of a fluorine atom in drug discovery can directly impact a molecule’s lipophilicity, modulate the acidity/basicity of neighboring functional groups, and improve potency [69]. Asymmetric fluorination reactions have therefore garnered significant attention from the synthetic community over the last 20 years [70]. In 2002, Kim reported the first example of enantioselective α‐fluorination of β‐ketoesters using phase‐transfer catalysis ( Scheme 4.18) [71]. A chiral cinchoninium catalyst was found to promote this reaction with moderate enantioselectivity using NFSI as the electrophilic fluorine source.
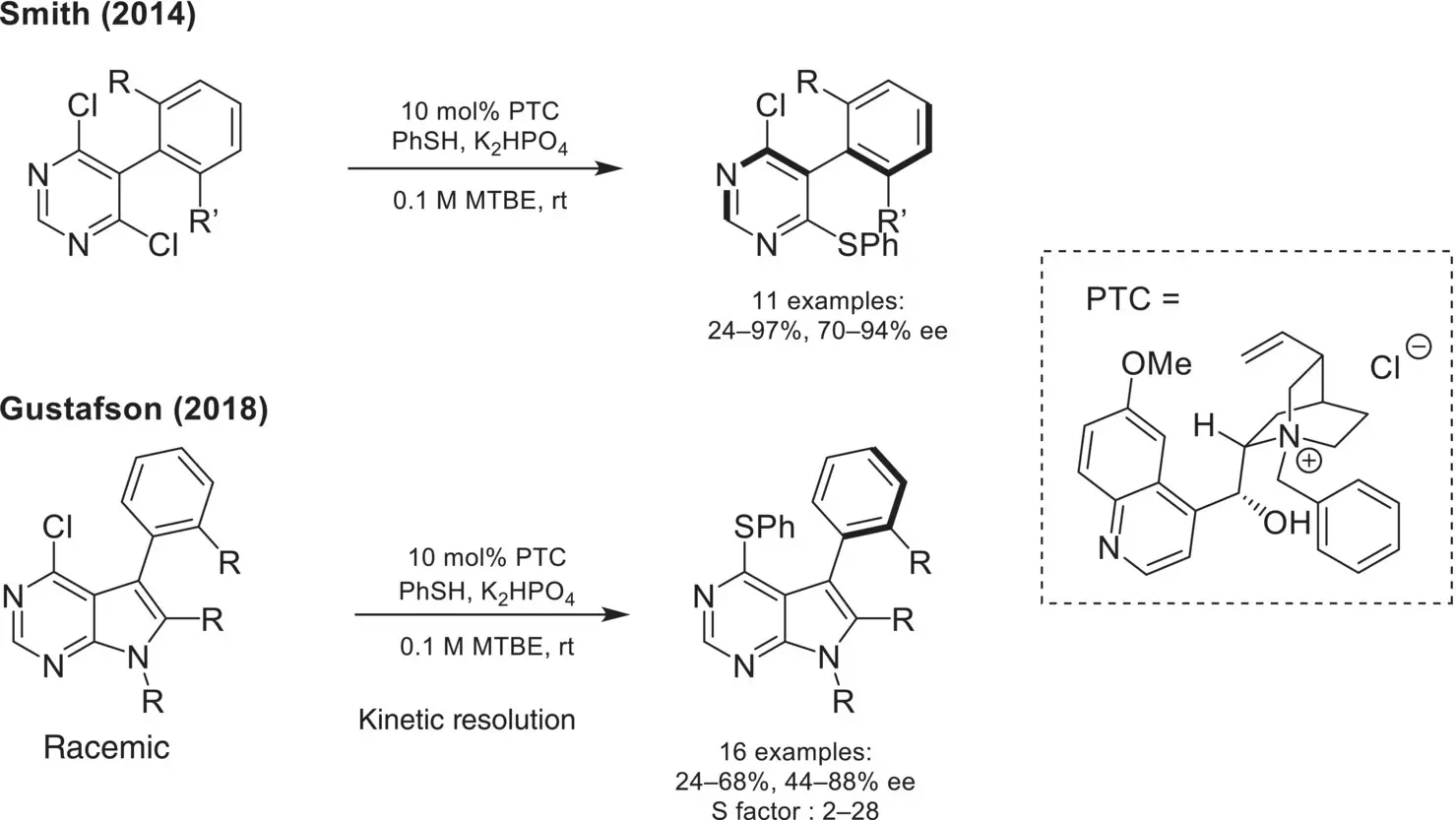
Scheme 4.16. Atropselective synthesis of biaryl motifs via nucleophilic aromatic substitution.
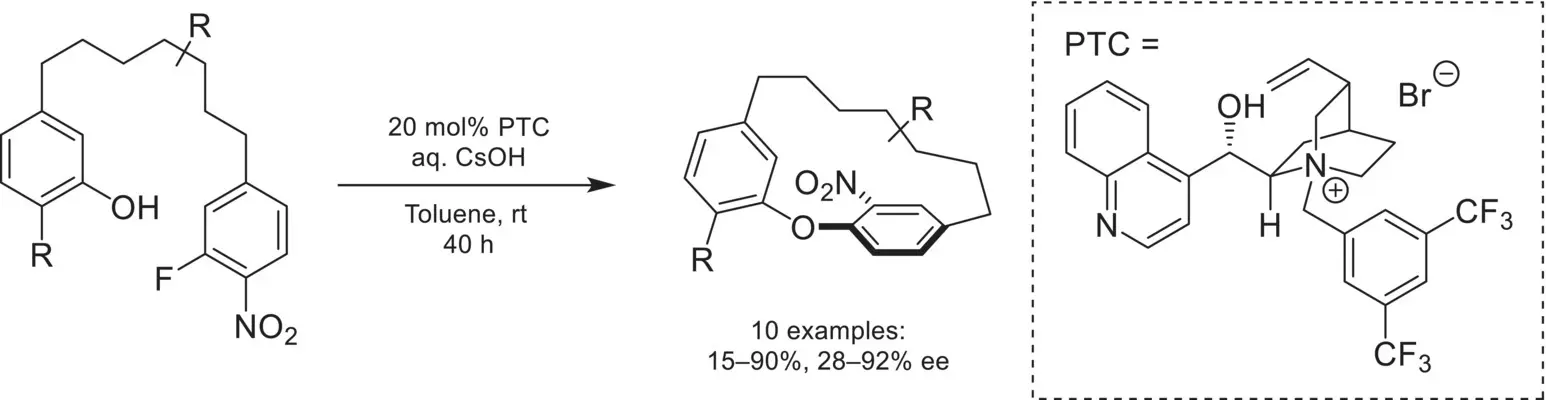
Scheme 4.17. Atropselective macrocyclization via nucleophilic aromatic substitution.
Source: Based on [68].
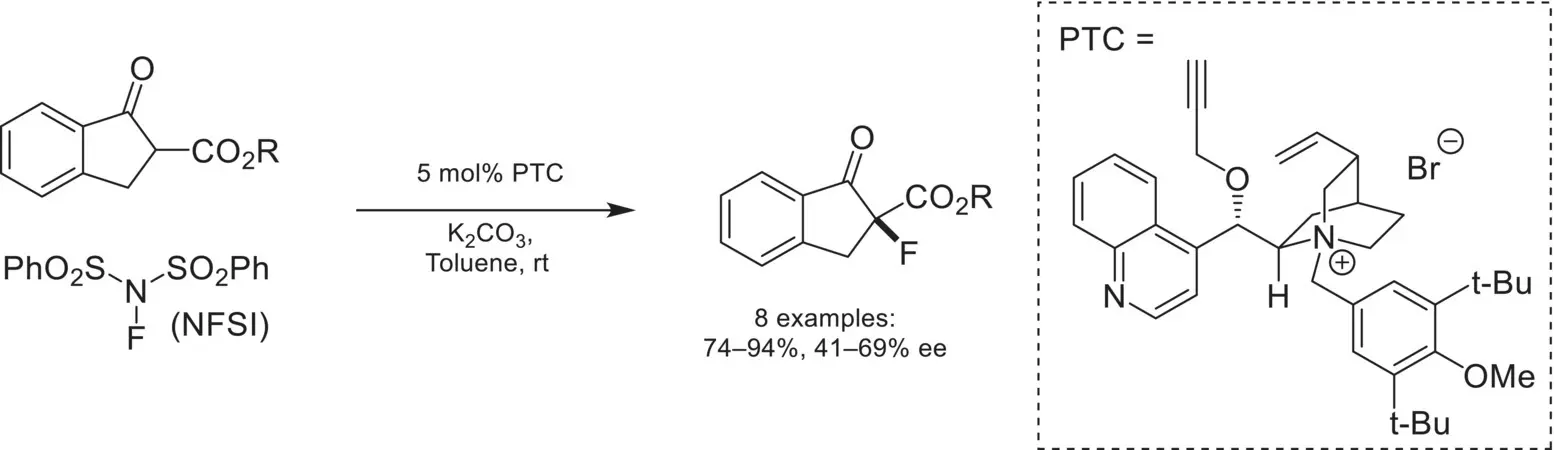
Scheme 4.18. Enantioselective α‐fluorination of β‐ketoesters.
Source: Based on [71].
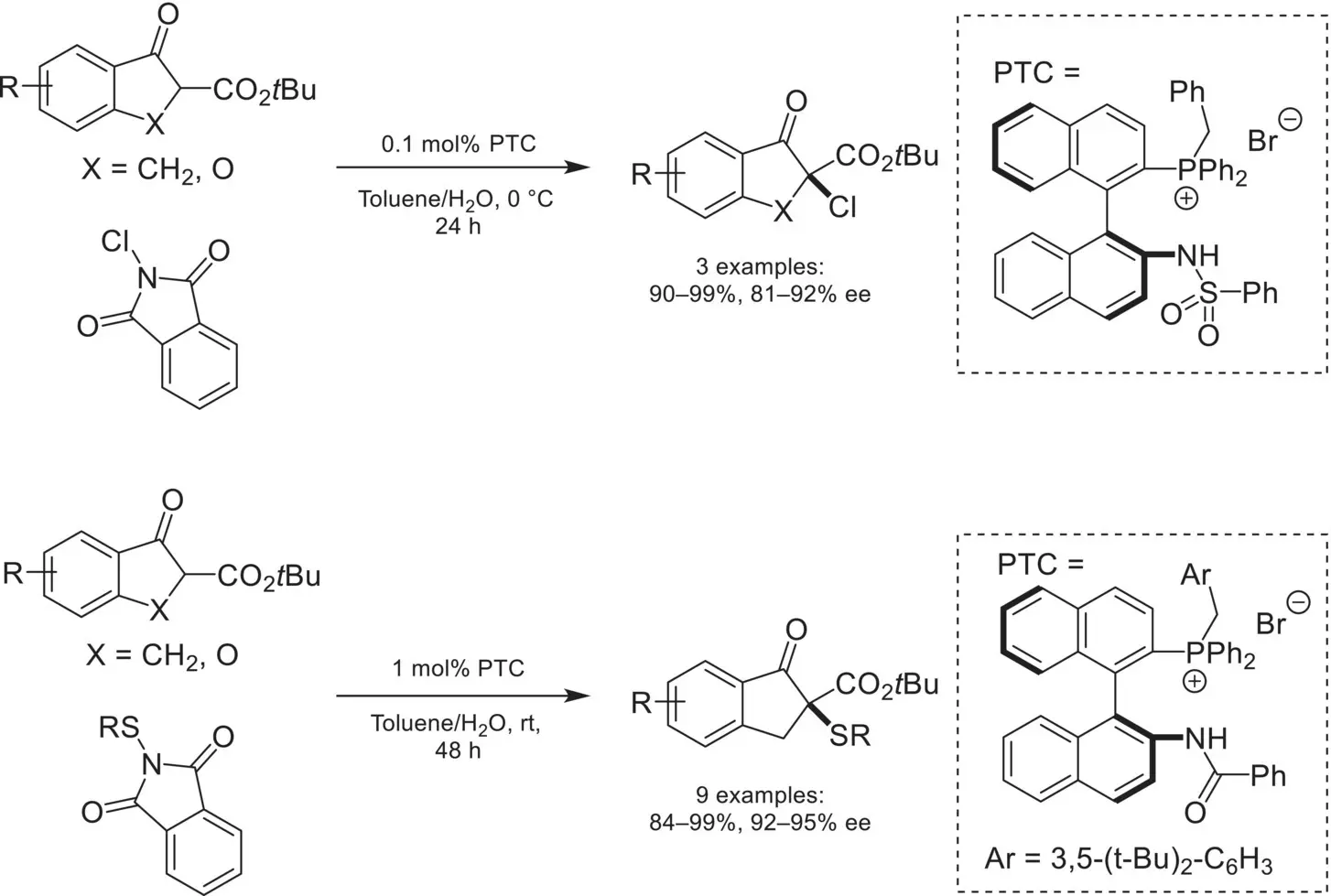
Scheme 4.19. Enantioselective α‐chlorination and α‐sulfenylation of 1,3‐dicarbonyl nucleophiles.
Source: Based on [72].
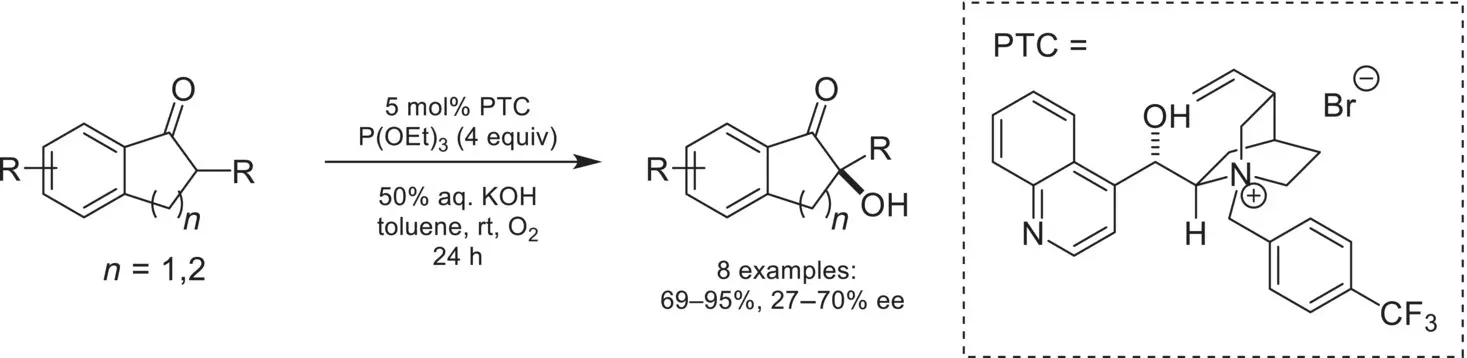
Scheme 4.20. Enantioselective α‐hydroxylation of indanones.
Source: Based on [73].
The α‐chlorination of enolates has also been reported using phase‐transfer catalysis, despite attracting less attention than α‐fluorination. In 2013, Maruoka reported the first α‐chlorination of β‐ketoesters using N‐chlorophthalimide and a chiral phosphonium‐based catalyst ( Scheme 4.19) [72]. Incorporation of a sulfonamide group on the binaphthyl backbone of the catalyst proved to be optimal. Interestingly, modification of the sulfonamide for a benzamide on the catalyst allowed the analogous α‐sulfenylation to proceed in high enantioselectivity using N‐(arylthiol)phthalimides as the electrophilic sulfenylation reagent.
The first example of enantioselective enolate α‐oxygenation was reported in 1988 by Shioiri ( Scheme 4.20) [73]. A chiral cinchoninium catalyst was found to promote the α‐oxygenation of indanones with moderate enantioselectivity from an in situ generated electrophilic hydroperoxide formed from triethylphosphite and molecular oxygen.
In 2008, 20 years after the seminal report by Shiori, Itoh disclosed a related system that proved to be highly enantioselective using an anthracene‐functionalized chiral cinchoninium catalyst ( Scheme 4.21) [74]. In the years following these reports, numbers of related systems have been reported using other electrophilic oxygenating reagents such as hydroperoxides or oxaziridines [75].
In 2008, Maruoka reported the first example of phase‐transfer catalyzed α‐amination of enolate derivatives. Specifically, β‐ketoesters could be aminated in high enantioselectivity using diazocarboxylates as electrophilic aminating reagents and a chiral tetraalkylphosphonium catalyst ( Scheme 4.22) [76]. Notably, this was a pioneering example of the use of a chiral tetraalkylammonium catalyst in phase‐transfer catalysis. The same group disclosed a year later that a more selective spirocyclic chiral ammonium catalyst could also promote this reaction at lower catalyst loadings [77]. A similar strategy was disclosed in 2011 by Ma [78] and later in 2019 by Maruoka [79] for the enantioselective amination of oxindoles and benzofuranones ( Scheme 4.23). Ma’s system used a quaternary phosphonium catalyst and Maruoka’s system used a spirocyclic ammonium catalyst, both featuring chiral C2‐symetric binaphthyl backbones.
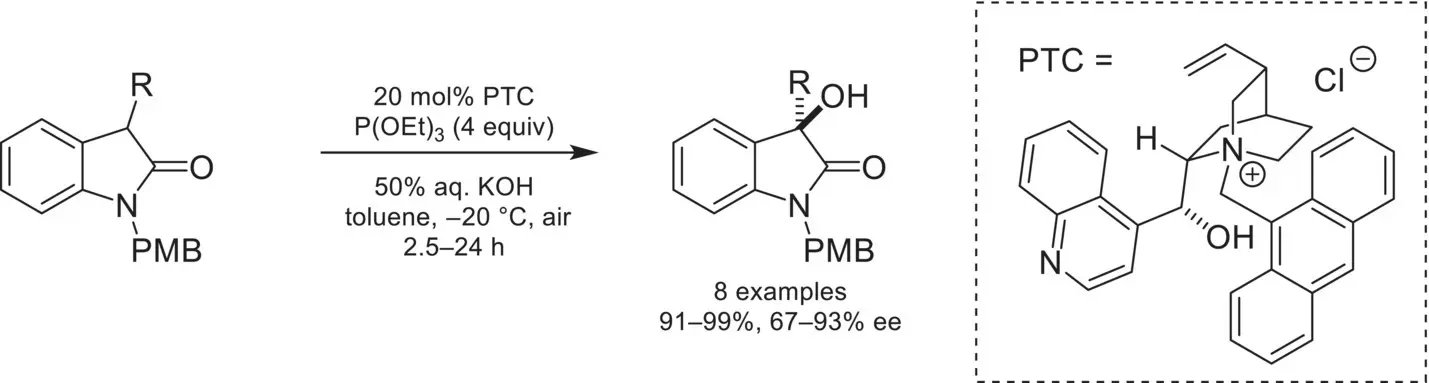
Scheme 4.21. Enantioselective α‐hydroxylation of oxindoles.
Source: Based on [74].
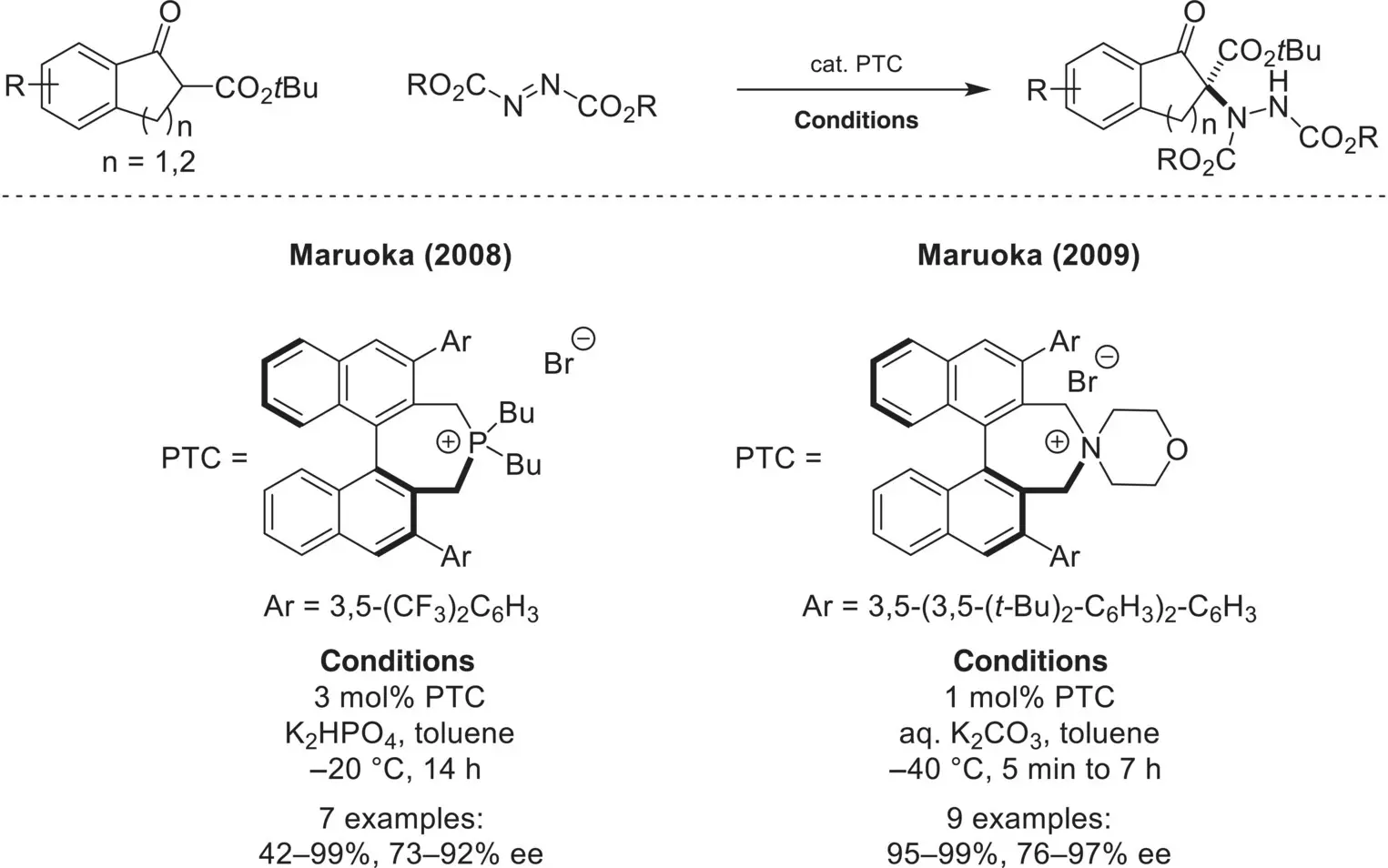
Scheme 4.22. Enantioselective α‐amination of β‐ketoesters using azodicarboxylates.
While diazocarboxylates proved to be suitable electrophilic amination reagents for phase‐transfer‐catalyzed enolate α‐amination to form hydrazine derivatives, subsequent reductive N–N bond cleavage is required to unmask the free amine. In 2016, Ooi disclosed an elegant system wherein direct amination is achieved using an electrophilic aminating reagent formed in situ from the corresponding hydroxylamine and trichloroacetonitrile ( Scheme 4.24) [80]. Notably, a triazolium‐based chiral phase‐transfer catalyst was optimal, providing α‐aminated oxindoles with high enantioselectivity.
Читать дальше
















