Philip Hofmann - Solid State Physics
Здесь есть возможность читать онлайн «Philip Hofmann - Solid State Physics» — ознакомительный отрывок электронной книги совершенно бесплатно, а после прочтения отрывка купить полную версию. В некоторых случаях можно слушать аудио, скачать через торрент в формате fb2 и присутствует краткое содержание. Жанр: unrecognised, на английском языке. Описание произведения, (предисловие) а так же отзывы посетителей доступны на портале библиотеки ЛибКат.
- Название:Solid State Physics
- Автор:
- Жанр:
- Год:неизвестен
- ISBN:нет данных
- Рейтинг книги:4 / 5. Голосов: 1
-
Избранное:Добавить в избранное
- Отзывы:
-
Ваша оценка:
- 80
- 1
- 2
- 3
- 4
- 5
Solid State Physics: краткое содержание, описание и аннотация
Предлагаем к чтению аннотацию, описание, краткое содержание или предисловие (зависит от того, что написал сам автор книги «Solid State Physics»). Если вы не нашли необходимую информацию о книге — напишите в комментариях, мы постараемся отыскать её.
Solid State Physics
Solid State Physics
Solid State Physics
Solid State Physics — читать онлайн ознакомительный отрывок
Ниже представлен текст книги, разбитый по страницам. Система сохранения места последней прочитанной страницы, позволяет с удобством читать онлайн бесплатно книгу «Solid State Physics», без необходимости каждый раз заново искать на чём Вы остановились. Поставьте закладку, и сможете в любой момент перейти на страницу, на которой закончили чтение.
Интервал:
Закладка:
The packing density of the cubic structure is improved in the body‐centered cubic(bcc) and face‐centered cubic(fcc) structures that are also depicted in Figure 1.4. In fact, the fcc structure has the highest possible packing density for identical spheres, as we shall see later. These two structures are very common – 17 elements crystallize in the bcc structure and 24 elements in the fcc structure. Note that the simple cubic structure ist the only one for which the cube is identical with the Bravais lattice. While the cube is also a unit cell for the bcc and fcc lattices, ist it not the primitive unit cell in these cases. Still, both structures are Bravais lattices with a basis containing one atom, but the vectors spanning these Bravais lattices are not the edges of the cube.
Cubic structures with a more complex basis than a single atom are also important. Figure 1.5shows the structures of the ionic crystals CsCl and NaCl, which are both cubic with a basis containing two atoms. For CsCl, the structure can be thought of as two simple cubic structures stacked into each other. For NaCl, it consists of two fcc lattices stacked into each other. Which structure is preferred for such ionic crystals depends on the relative size of the positive and negative ions.
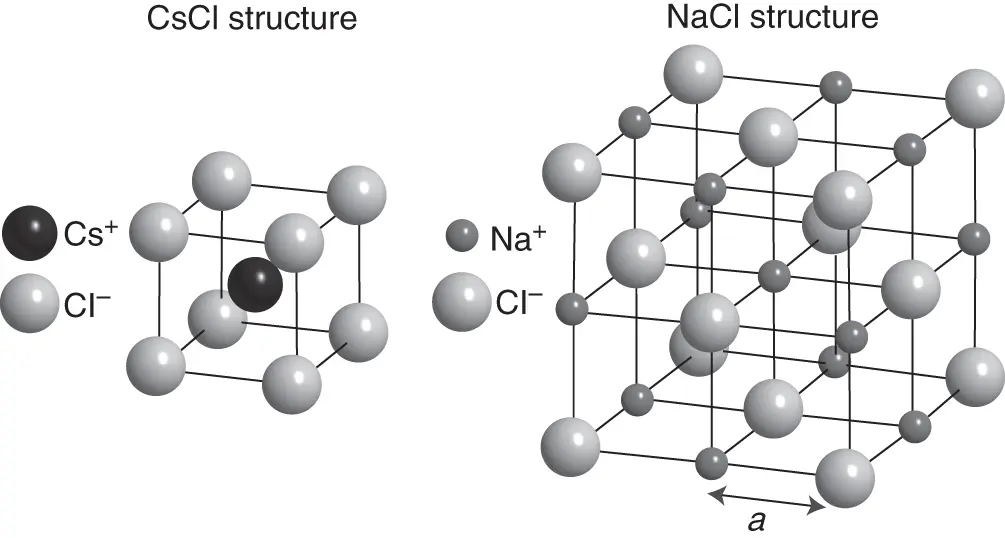
Figure 1.5 Structures of CsCl and NaCl. The spheres are depicted much smaller than in the situation of dense packing, but the relative size of the different ions in each structure is correct.
1.2.2 Close‐Packed Structures
Many metals prefer structural arrangements where the atoms are packed as closely as possible. In two dimensions, the closest possible packing of atoms (i.e. spheres) is the hexagonal structure shown on the left‐hand side of Figure 1.6. To build a three‐dimensional close‐packed structure, one adds a second layer as in the middle of Figure 1.6. Now there are two possibilities, however, for adding a third layer. We can either put the atoms in the “holes” just on top of the first‐layer atoms, or we can put them into the other type of “holes.” The result are two different crystal structures. The first has an ABABAB… layer stacking sequence, the second an ABCABCABC… layer stacking sequence. Both have exactly the same packing density with the spheres filling about 74% of the total volume. The former structure is called the hexagonal close‐packed structure(hcp), and the latter turns out to be the fcc structure we already know. An alternative sketch of the hcp structure is shown in Figure 1.16b. The fcc and hcp structures are very common in elemental metals, 36 chemical elements crystallizing in hcp and 24 in fcc lattices. These structures also maximize the number of nearest neighbors for a given atom, the so‐called coordination number. For both the fcc and the hcp lattices, the coordination number is 12.
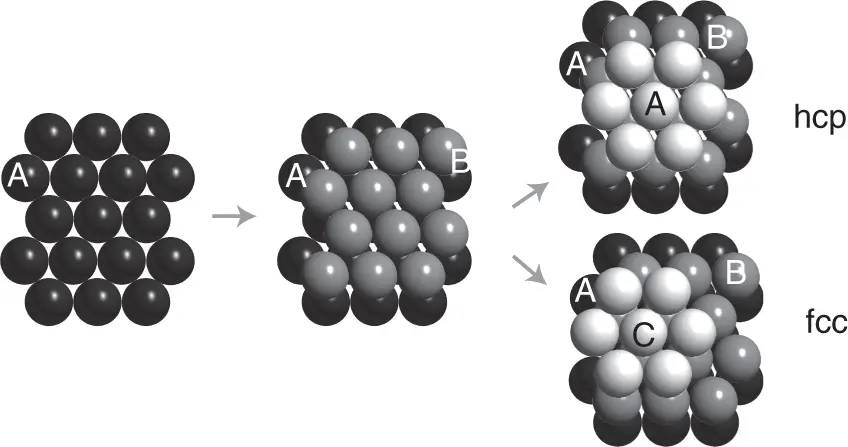
Figure 1.6 Close packing of spheres leading to the hcp and fcc structures.
It is as yet an unresolved question why not all metals crystallize in the fcc or hcp structures, if coordination is indeed so important. Whereas a prediction of the actual structure for a given element is not possible on the basis of simple arguments, we can identify some factors that play a role. For example, structures that are not optimally packed, such as the bcc structure, have a lower coordination number, but they bring the second‐nearest neighbors much closer to a given atom than in the close‐packed structures. Another important consideration is that the bonding situation is often not quite so simple, particularly in transition metals. In these, bonding is not only achieved through the delocalized s and p valence electrons as in simple metals, but also by the more localized d electrons. Bonding through the latter results in a much more directional character so that not only the close packing of the atoms is important.
The structures of many ionic solids can also be viewed as “close‐packed” in some sense. One can derive these structures by treating the ions as hard spheres that have to be packed as closely to each other as possible.
1.2.3 Structures of Covalently Bonded Solids
In covalent structures, the valence electrons of the atoms are not completely delocalized but shared between neighboring atoms, and bond lengths and directions are far more important than the packing density. Prominent examples are graphene, graphite, and diamond as displayed in Figure 1.7. Graphene is a single sheet of carbon atoms in a honeycomb lattice structure. It is a truly two‐dimensional solid with a number of remarkable properties – so remarkable, in fact, that their discovery has lead to the 2010 Nobel prize in physics being awarded to A. Geim and K. Novoselov. The carbon atoms in graphene are connected through a network of sp  hybrid bonds enclosing angles of 120
hybrid bonds enclosing angles of 120  . The parent material of graphene is graphite, which consists of a stack of graphene sheets that are weakly bonded to each other. In fact, graphene can be isolated from graphite by peeling off flakes with a piece of scotch tape. In diamond, the carbon atoms form sp
. The parent material of graphene is graphite, which consists of a stack of graphene sheets that are weakly bonded to each other. In fact, graphene can be isolated from graphite by peeling off flakes with a piece of scotch tape. In diamond, the carbon atoms form sp 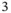 ‐type bonds and each atom has four nearest neighbors in a tetrahedral configuration. Interestingly, the diamond structure can also be described as an fcc Bravais lattice with a basis of two atoms.
‐type bonds and each atom has four nearest neighbors in a tetrahedral configuration. Interestingly, the diamond structure can also be described as an fcc Bravais lattice with a basis of two atoms.
Figure 1.7 Structures for (a) graphene, (b) graphite, and (c) diamond. Bonds from sp  and sp
and sp  orbitals are displayed as solid lines.
orbitals are displayed as solid lines.
The diamond structure is also found for Si and Ge. Many other isoelectronic materials (i.e. materials with the same total number of valence electrons), such as SiC, GaAs, or InP, also crystallize in a diamond‐like structure but with each element on a different fcc sublattice.
1.3 Crystal Structure Determination
After having described different crystal structures, the question is of course how to determine these structures in the first place. By far the most important technique for this is X‐ray diffraction. In fact, the importance of this technique extends far beyond solid state physics, as it has become an essential tool for fields such as structural biology as well. In biology, the idea is that you can derive the structure of a given protein by trying to crystallize it and then use the powerful methodology of X‐ray diffraction to determine its structure. In addition, we will also use X‐ray diffraction as a motivation to extend our formal description of structures.
1.3.1 X‐Ray Diffraction
Интервал:
Закладка:
Похожие книги на «Solid State Physics»
Представляем Вашему вниманию похожие книги на «Solid State Physics» списком для выбора. Мы отобрали схожую по названию и смыслу литературу в надежде предоставить читателям больше вариантов отыскать новые, интересные, ещё непрочитанные произведения.
Обсуждение, отзывы о книге «Solid State Physics» и просто собственные мнения читателей. Оставьте ваши комментарии, напишите, что Вы думаете о произведении, его смысле или главных героях. Укажите что конкретно понравилось, а что нет, и почему Вы так считаете.












