1 ...7 8 9 11 12 13 ...29 The XerCD system is versatile and is used in site‐specific recombination reactions with sequences related, but not identical, to dif , and accompanied by co‐factor proteins, to resolve dimers in autonomously replicating plasmids (Clerget 1991; Colloms 2013; Colloms et al. 1990, 1998; Summers 1989). In the human pathogen V. cholerae , the CTXφ bacteriophage that carries the cholera toxin ctxAB operon integrates into chromosome I at its dif site using the XerC recombinase from the V. cholerae XerCD recombinases to catalyse the reaction (it can enter the corresponding location on chromosome II at a lower frequency) (Das et al. 2013; Huber and Waldor 2002; McLeod and Waldor 2004). CTXφ is a filamentous phage and only the plus strand of its genome integrates. The plus strand of the CTXφ genome folds to create a double‐stranded region that encompasses the XerC and XerD binding sites of the phage flanking a mismatched and bulging phage dif site. Only a single stranded exchange occurs, mediated by XerC alone, and this creates a Holliday junction that is resolved by DNA replication (Val et al. 2005). The minus strand of the CTXφ, phage fails to generate a dif site with enough homology to recombine with the chromosomal counterpart, so this strand of the phage genome does not integrate. The integrated phage does not excise because its dif sites also lack sufficient homology with the chromosomal site to promote site‐specific recombination (Val et al. 2005).
1.9 Segregating the Products of Chromosome Replication
The daughter chromosomes have to be moved to locations in the mother cell that correspond to the emerging daughter cells. At the end of the movement period, it should be possible to close the cell division septum between the nascent daughter cells without damaging the chromosome copies by guillotining them. The chromosome segregation process follows the Origin‐to‐Terminus axis, just as the replication process does (Bouet et al. 2014) ( Figure 1.9).
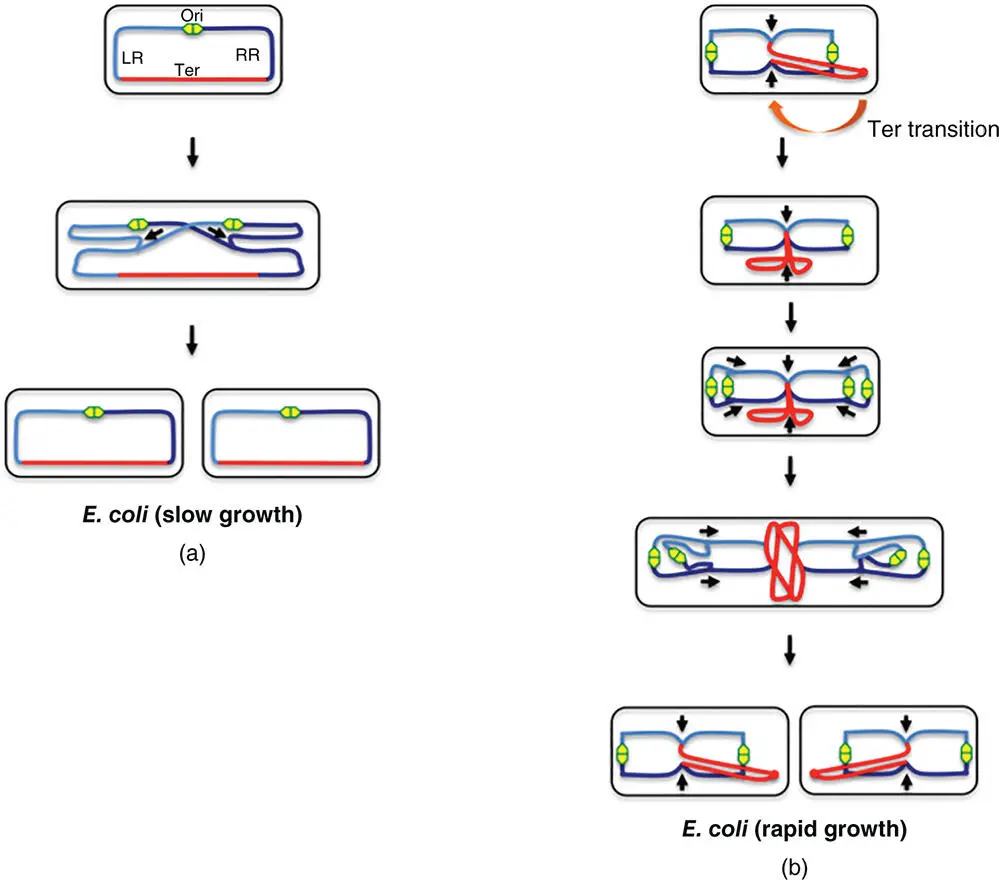
Figure 1.9The choreography of chromosome movement in E. coli during the cell cycle. (a) In slow‐growing E. coli cells, chromosome replication is initiated at the origin (Ori, green/yellow lozenge) located at mid‐cell, and proceeds bidirectionally, copying the left (LR, light blue) and right (RR, dark blue) replichores and ending in the terminus (red), where the products of replication are decatenated prior to segregation into the daughter cells. Arrows drawn alongside the chromosome are used to indicate the direction of replication fork movement. (b) Rapidly growing E. coli cells have multiple rounds of chromosome replication underway simultaneously. Instead of the mid‐cell position seen in slow‐growing E. coli cells, the Ori is at the cell pole in the rapidly growing bacteria. The newly born cell has its Ter region displaced towards one pole of the cell and this undergoes a transition to the mid‐cell. A second round of chromosome replication starts before the previous one is complete and multiple replication forks can be observed. The final separation of the daughter chromosomes is thought to exert a force at the terminus that moves this part of the chromosome to an eccentric position that is maintained in the daughter cell immediately after its birth (Youngren et al. 2014).
The immediate products of replication behind the moving fork and replisome are catenated, positively supercoiled, interwound, double‐stranded DNA molecules. Topo IV will attempt to decatenate these (Deibler et al. 2001; Espéli et al. 2003; Khodursky et al. 2000; Lopez et al. 2012). However, in a 300‐ to 400‐kb sliding window immediately in the wake of fork passage, the DNA is only hemimethylated. Any hemimethylated SeqA sites can bind the SeqA protein, which has the potential both to bridge DNA molecules and to exclude Topo IV, preventing decatenation (Joshi et al. 2013). The result is cohesion of the chromosome copies (Joshi et al. 2011; Nielsen et al. 2006a).
Two factors are thought to contribute to cohesion: exclusion of Topo IV by SeqA and bridging of daughter chromosomes by SeqA bound to hemimethylated copies of its binding sites. In support of the model, a positive correlation has been described between the time delay in ending cohesion and the density of SeqA‐binding sites at oriC and at two so‐called ‘snap’ loci (in the Right Replichore in E. coli ) at which particularly strong physical tethers seem to fail suddenly as the replication‐and‐segregation process proceeds (Joshi et al. 2011). Chromosome cohesion must be overcome if segregation is to proceed to completion and this involves efficient decatenation of interwound chromosome copies and the breaking of any inter‐chromosome bridges. The resulting segregation process appears to include a series of successive jerks as each tether breaks in turn (Fisher et al. 2013). Dam‐mediated methylation of the newly synthesised DNA strands seems to be a key step in preventing SeqA binding and using its capacity to bridge chromosome copies and to interfere with Topo IV access to catenated DNA substrates behind the fork (Joshi et al. 2011). Cohesion does not require the Structural Maintenance of Chromosome (SMC) proteins, such as MukBEF in E. coli , despite their potential to encircle DNA duplexes (Adachi et al. 2008; Danilova et al. 2007; Joshi et al. 2013) ( Figure 1.10).
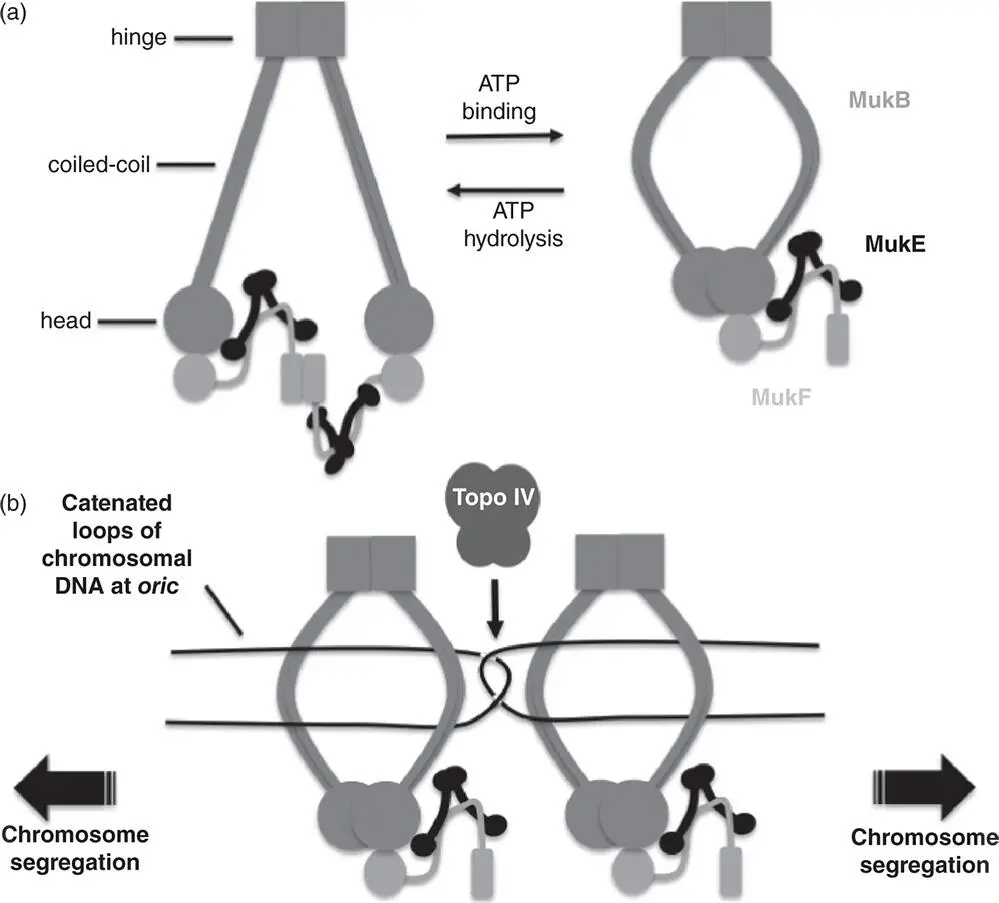
Figure 1.10MukBEF structure and function. (a) The Dimeric MukB protein (intermediate grey) consists of a head domain with ATPase activity that is attached to a hinge domain by a coiled‐coil. A ‘cap’ region in the head domain interacts with carboxyl terminus of MukF (light grey) while the central segment of MukF interacts with a MukE dimer (black). When ATP is absent, the complex has two copies of MukB, MukF and four of MukE. One MukF protein is displaced following ATP binding by MukB. This form of the MukBEF complex may in turn dimerise (not shown) (Nolivos and Sherratt 2014). (b) Clusters of MukBEF complexes, here represented by just two, have the potential to organise and segregate the ori regions of sister chromosomes following replication. The catenated ori regions are decatenated by Topo IV (dark grey), working in combination with the DNA segregation activity of the MukBEF motor.
The presence or absence of a ParAB‐ parS system is a major determinant of the pattern of chromosome segregation seen in a bacterium. E. coli does not possess such a system and forces of mutual repulsion acting on the chromosome copies as they emerge in the confined space of the rod‐shaped cell may drive them to segregate, perhaps aided by the imprinted structural and super‐structural features of the chromosomes (Jun and Mulder 2006; Jun and Wright 2010; Junier et al. 2014; Pelletier et al. 2012; Wiggins et al. 2010).
ParAB‐ parS systems may be useful rather than essential in bacteria that have just one chromosome, unless the bacterium is sporulating (Ireton et al. 1994) or going through a growth phase transition (Godfrin‐Estevenon et al. 2002). If the microbe has more than one chromosome, then the partitioning system is essential if the segregation of its different chromosomes is to be properly coordinated, as, for example, in the case of V. cholerae (Yamaichi et al. 2007) or members of the Burkholderias (Passot et al. 2012). The roles of chromosome‐encoded ParAB‐ parS systems as functioning partitioning machines was confirmed in early work where it was demonstrated that they could replace the native plasmid par systems on single copy episomes (Godfrin‐Estevenon et al. 2002; Lin and Grossman 1998; Yamaichi and Niki 2000).
Читать дальше