Conversion of DnaA‐ADP to DnaA‐ATP has been associated with two so‐called DnaA Reactivation Sites, DARS1 and DARS2 (Fujimitsu et al. 2009) ( Figure 1.5). DARS1 is 103 bp in length, has three DnaA binding sites, and is located upstream of uvrB in E. coli . The DARS2 site is more sophisticated. It is 455 bp in length and is located upstream of the mutH gene in E. coli . DARS2 binds IHF and FIS in addition to DnaA. Binding of these NAPs to DARS2 stimulates the conversion of DnaA‐ADP to DnaA‐ATP. IHF binding is cell cycle determined while FIS binding is growth phase determined: FIS binds in rapidly growing cells and this is consistent with the observation that FIS stimulates DNA replication in rapidly growing E. coli (Flåtten and Skarstad 2013; Kasho et al. 2014). The chromosomal locations of datA and the DARS elements seem to be important for their function: if they are repositioned, the chromosome replication control is disrupted (Frimodt‐Møller et al. 2016).
The oriC locus is found between two highly conserved genes, mioC and gidA ( Figure 1.6). The mioC gene is transcribed towards oriC while gidA is transcribed away from it. The two genes exhibit reciprocal transcription patterns that are functions of the cell cycle: mioC transcription is maximal midway through chromosome replication while gidA transcription is minimal at that point; maximal expression of gidA coincides with the onset of septation and cell division (Lies et al. 2015). MraZ, a protein possibly involved in cell division control, binds and represses the mioC promoter (Eraso et al. 2014) and this promoter is also stringently regulated, linking mioC transcription to growth rate (Chiaramello and Zyskind 1989). The biological function of MioC is not firmly established, although it has been reported to be involved in biotin metabolism (Birch et al. 2001). The GidA protein contributes to tRNA modification, working in association with MnmE (GidA is also known as MnmG) (Bregeon et al. 2001). Neither protein is thought to have DNA‐binding activity. Transcription of mioC is repressed by DnaA acting at a DnaA box in the promoter. The initiation of chromosome replication displaces DnaA and de‐represses mioC , with the return of DnaA being delayed as the protein is recruited by the new DnaA binding sites generated by replication (Bogan and Helmstetter 1996). Transcription of gidA is repressed by SeqA when this protein binds to the 5′‐GATC‐3′ sites at the promoter that become hemimethylated following DNA replication (Bogan and Helmstetter 1997). The process of transcribing gidA and mioC is important for the initiation of chromosome replication at oriC (Bramhill and Kornberg 1988b; Theisen et al. 1993), at least under some circumstances (Asai et al. 1998; Bates et al. 1997; Lies et al. 2015).
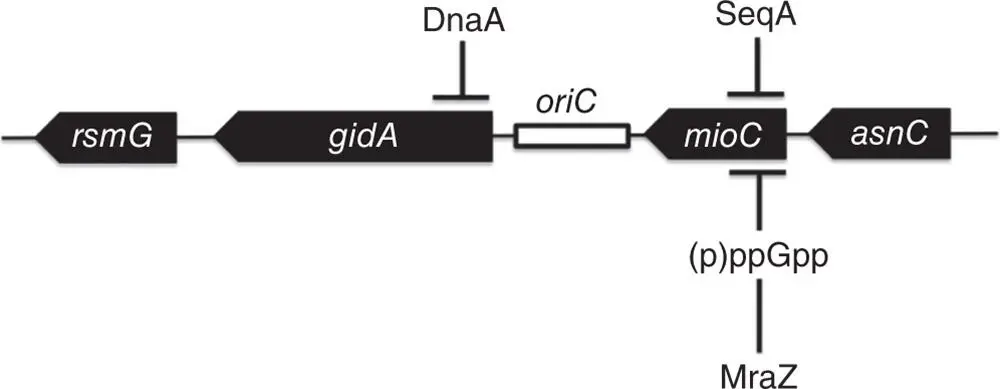
Figure 1.6The genetic neighbourhood of oriC in E. coli . Filled arrows represent the genes and an open rectangle indicates the position of oriC . DnaA represses the gidA gene transcriptionally through DnaA boxes that overlap the gidA promoter. The mioC gene is repressed by SeqA binding to hemimethylated versions of 5′‐GATC‐3′ sites at the promoter that are generated by DNA replication. The mioC promoter is also subject to stringent control via the (p)ppGpp alarmone and it is repressed by MraZ, a protein that has been linked to the control of cell division. The rsmG gene encodes a methyltransferase for the modification of 16S rRNA (see Benítez‐Páez et al. 2012). The asnC gene encodes a HTH‐motif‐containing transcription regulator that is related to LRP and controls genes involved in asparagine metabolism (see Kölling and Lother 1985; Willins et al. 1991). Termination of transcription extending from asnC to mioC is dependent on a DnaA‐DNA complex at the asnC terminator, as described by Schaefer and Messer (1988).
1.4 Chromosome Replication: Elongation
Once replication has been initiated, the replisome is responsible for progressive DNA synthesis during the elongation phase of chromosome replication. This large complex is composed of a pentameric clamp loader, the DNA polymerase clamp (DnaN), the three‐subunit DNA primase (DnaG), and the hexameric helicase DnaB (Bailey et al. 2007; Reyes‐Lamothe et al. 2010) ( Figure 1.4). The helicase uses ATP hydrolysis to unwind the DNA duplex, moving along the lagging strand of the DNA as it does so. Single‐stranded DNA‐binding protein (SSB) coats the separated ssDNA strands, thus preventing reformation of the duplex by religation and attack by nucleases (Beattie and Reyes‐Lamothe 2015).
The primase, DnaG, possesses a central RNA polymerase domain where the RNA primers used in DNA synthesis are manufactured (Corn et al. 2008). The primer emerges from the DnaG‐DnaB complex and is transferred to DNA polymerase and SSB (Corn et al. 2008). DNA Polymerase III works with the beta‐clamp protein (DnaN) to extend the primer, creating a new DNA strand at a rate of 1000 bases per second (Beattie and Reyes‐Lamothe 2015). It is advantageous to have DnaN as a component of the replisome because a beta‐clamp must be reloaded for the synthesis of each lagging strand Okazaki fragment (Beattie and Reyes‐Lamothe 2015). If the replication fork stalls or breaks, replication can be restarted through a DnaA‐independent mechanism. Here, the PriA helicase, in association with accessory proteins such as PriB, PriC, and DnaT, binds to the gapped replication fork and loads DnaBC. In some cases, the restart may be associated with a strong transcription promoter that generates an R‐loop where PriA can introduce DnaBC on the displaced DNA strand (Heller and Marians 2006; Kogoma 1997). Of the approximately 300 copies of DNA gyrase that are bound to the E. coli chromosome at any one time, about 12 accompany each moving replication fork to manage the DNA topological disturbance that is associated with fork migration (Stracy et al. 2019).
1.5 Chromosome Replication: Termination
Termination of DNA synthesis occurs within Ter, located at a point that is diametrically opposite oriC on the chromosome (Hill et al. 1987) ( Figure 1.7). The Ter region has five copies of a 23‐bp DNA element on each flank and the 36‐kDa Tus protein binds to these sequences (Neylon et al. 2005). The Tus binding sites are asymmetric and have a permissive and a non‐permissive orientation ( Figure 1.7). Replication forks can pass the Tus‐Ter nucleoprotein complexes when the DNA sequences are in the permissive orientation, but fork movement becomes arrested when the sequences are oriented in the non‐permissive direction. The mechanism of replication fork passage at Ter sites that are in the permissive orientation involves displacement of Tus by the DnaB helicase; when in the non‐permissive orientation, Tus prevents DnaB, and the replication fork, from translocating past that point (Bastia et al. 2008; Berghuis et al. 2015; Mulcair et al. 2006). Single‐molecule experiments performed in vitro have shown that the DNA also plays a critical role: in the non‐permissive orientation, the unwinding of the DNA by the approaching replication fork creates a powerful lock at the Tus‐Ter site that is an effective roadblock to further translocation by the fork; in the permissive orientation the lock does not operate and the fork can proceed (Berghuis et al. 2015).
Читать дальше