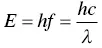Source Wikipedia, https://en.wikipedia.org/wiki/Johann_Jakob_Balmer#/media/File:Balmer.jpeg (left); Wikipedia, https://en.wikipedia.org/wiki/Johannes_Rydberg#/media/File:Rydberg,_Janne_(foto_Per_Bagge;_AFs_Arkiv).jpg (right).
Albert Einstein (1879–1955, Figure 1.8) published a paper in 1905 on the theory of the photoelectric effect. When light strikes a metal surface, it frees an electron if its energy is higher than a given threshold value. Any remaining energy is used to kick the electron off the surface. In his paper, Einstein proposed the concept that light has a dual personality; it behaves like a wave or like a particle, and the particle has an energy associated with the wavelength of that light.
He called this particle a “light quantum.” (In 1926, a French physicist named Frithiof Wolfers [1891–1971] renamed the light quantum a photon . It is interesting that Einstein received the Nobel Prize in 1921 for the discovery of the photon, not for his much more famous work on relativity.) This light particle, the photon, has an energy that depends on the frequency of the light. The energy associated with this light is given by the formula
(1.5) 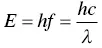
where h is Planck's constant ( h = 6.63 10 −34m 2kg s −1), c is the speed of light ( c = 3 × 10 8m s −1), and λ is the wavelength (m). The meter in the numerator cancels the one in the denominator, resulting in the energy given in Joules (= kg m 2s −2).

Figure 1.8 Around 1905, Albert Einstein came up with the concept that light behaves as both a wave and a particle.
Source: Wikipedia, https://en.wikipedia.org/wiki/Albert_Einstein#/media/File:Einstein_patentoffice.jpg.
While all of these light experiments and relationships were being observed in the late nineteenth century, other scientists were playing with cathode‐ray tubes, the precursors of old television sets and oscilloscopes, trying to understand the nature of the atom. The cathode‐ray tube consists of an evacuated tube with two contacts, one at each end: the cathode and the anode . When a voltage is applied across the tube, current flows from the cathode to the anode, and the tube glows. The scientists explained this phenomenon by saying that electrons going through an evacuated tube containing very few atoms are able to attain sufficient velocity (and therefore kinetic energy) to hit the atoms and make them glow. They were called cathode rays .
Nobel Prize winning British physicist Joseph John Thomson (1856–1940, Figure 1.9) studied cathode rays and postulated in 1897 that they consisted of extremely small negatively charged particles, which he initially called “corpuscles.” (As happened with the term photon , George Stoney (1826–1911) later renamed corpuscles as electrons .) By studying how these particles moved through the gas and how they could be deflected by magnets, Thomson concluded that the “corpuscles” were (i) negatively charged particles and (ii) much smaller than the atoms themselves – at least 1000 times smaller. To account for electrically neutral atoms, he proposed that there is a core of positive charges with a large mass surrounded by an amorphous cloud of negatively charged electrons.
Ernest Rutherford (1871–1937, Figure 1.10), also a Nobel Prize winner, worked with radioactivity. In 1911, he bombarded very thin gold foil with alpha particles and looked at the scattered reflections as the radiation went through the foil. Most of the radiation went through the foil undeflected. Only a few alpha particles were reflected back and, from the angle of the reflected radiation, he concluded that the atom must have a very small, concentrated, positively charged core to compensate for the negatively charged electrons. Because the large majority of alpha particles passed through the foil without any directional change, he concluded that the majority of the space in an atom is empty, and the electrons are orbiting the nucleus instead of just being a scrambled negatively charged cloud as Thomson had suggested.
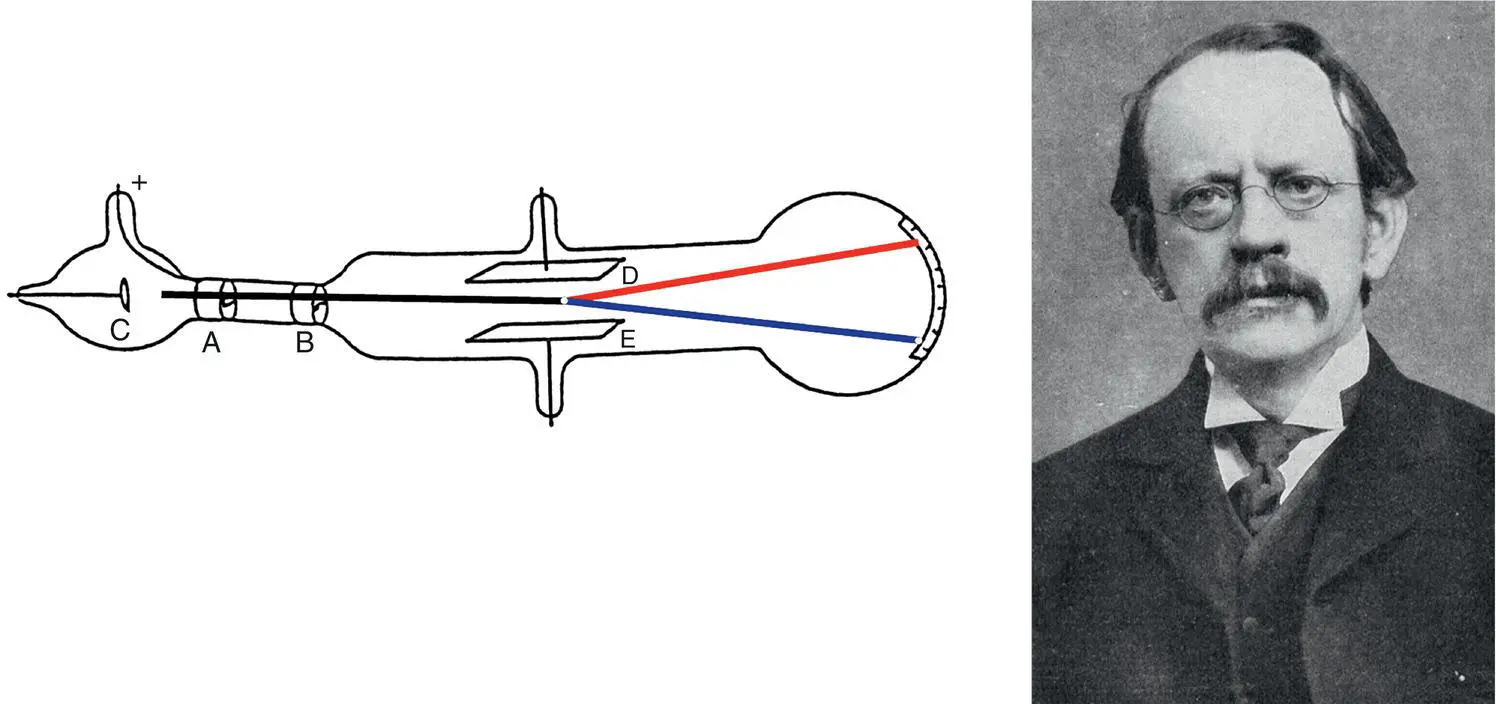
Figure 1.9 Joseph John Thomson and his cathode ray tube.
Source: Wikipedia, https://en.wikipedia.org/wiki/J._J._Thomson#/media/File:JJ_Thomson_Cathode_Ray_2.png (left); Wikipedia, https://en.wikipedia.org/wiki/J._J._Thomson#/media/File:J.J_Thomson.jpg (right).
Figure 1.10 Ernest Rutherford, with his experiment that bombarded alpha particles with radiation, concluded that the nucleus is extremely small and is concentrated at the center of the atom.
Source: Wikipedia, https://upload.wikimedia.org/wikipedia/commons/6/6e/Ernest_Rutherford_LOC.jpg.
Robert Millikan (1868–1953, Figure 1.11) was able to measure the electrical charge of an electron with an interesting oil drop experiment in 1909. He suspended a very small charged oil drop between two metal plates – one positive and the other negative – creating an electric field between the plates. He dropped tiny oil droplets into a vacuum chamber, and with X‐rays, he negatively charged some of the oil drops. By changing the electric field between the two plates, he could control the speed of the oil drops, slowing them down, stopping them, or even moving them upward. By knowing the density of the oil drop, the size of the drop, its volume and mass, and the electric field that compensated for the effect of gravity, he was able to come up with the value of the charge of a single electron: 1.592 × 10 −19coulombs (he was off by less than 1% of the now‐established number – not bad at all).
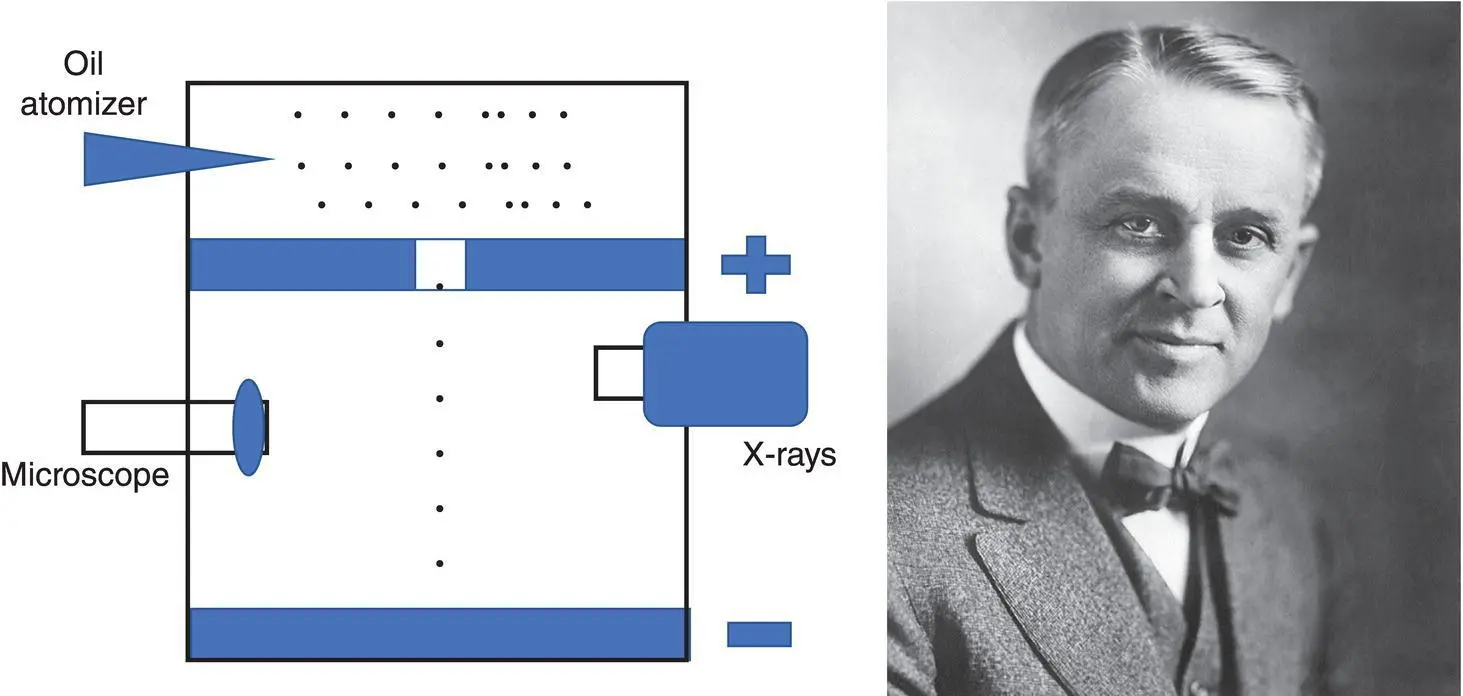
Figure 1.11 Robert Millikan, with his oil‐drop experiment, measured the electrical charge of an electron.
Source: https://en.wikipedia.org/wiki/Robert_Andrews_Millikan#/media/File:Millikan.jpg.
So here we are in 1913 (just a mere 105 years ago at the time of this writing). What did Bohr know? He knew:
1 That a hydrogen atom is the simplest atom, consisting of just one proton (positively charged) and one electron (negatively charged).
2 That all of the atom's mass is concentrated at the core: that is, the proton.
3 That electrons are negative particles somehow orbiting the nucleus.
4 That the great majority of space in an atom is empty.
5 That all the other elements can be organized neatly by weight on a periodic table.
6 That all elements have different emission spectra with specific emission or absorption color lines.
Niels Bohr (1885–1962, Figure 1.12) was able to beautifully explain all of these observations and how the spectral lines are generated. He postulated in 1912 that an atom consists of a core nucleus that has all the mass and is surrounded by electrons, moving like a planetary system in well‐defined orbits ( Figure 1.13). Electrostatic forces between the proton and the electron (analogous to the gravitational forces in the solar system) keep the electrons circulating without escaping their orbit. Additionally, Bohr postulated that the electrons in orbit do not radiate any energy, so the orbits are stable. The only way to radiate or absorb any energy is for an electron to jump from one orbit to another, and that is precisely what explains the spectra of hydrogen and other elements.
Читать дальше