Figure 2.15illustrates a current model for how ρ terminates transcription. The ρ protein forms a hexameric (six-member) ring made up of six subunits encoded by the rho gene. This ring binds to a sequence in the RNA called the rut (for rho utilization) site. However, ρ can bind to a rut site in the RNA only if the RNA in this region is not occupied by ribosomes during translation of an mRNA; for example, ρ can bind if translation has terminated upstream at a termination codon (see below). The rut sites are not very distinctive but are about 40 nucleotides long and have many C’s and have minimal secondary structure in the mRNA. Once ρ has bound to a rut site through the outside of its ring, the RNA then passes through the central hole, and the ring then moves along the RNA in the 5′-to-3′ direction, following the RNA polymerase. Energy for this movement is provided by the cleavage of ATP to adenosine diphosphate (ADP) by the ATPase activity of ρ. The ρ factor ring rotates down the RNA behind the RNA polymerase at a speed of about 60 nucleotides per second. However, RNA polymerase is capable of transcribing at 100 nucleotides per second, so ρ factor can catch up only if the RNA polymerase pauses at a ρ-dependent termination site. Then, the ρ factor can catch up to the RNA polymerase, and its RNA-DNA helicase activity disrupts the RNA-DNA hybrid in the transcription bubble, stopping transcription and releasing the RNA polymerase from the DNA template. While this model accounts for most of the known activities of ρ, it leaves unanswered the question of how ρ can access the RNA-DNA helix, which is inside the RNA polymerase. One possibility is that the RNA polymerase partially opens up when it is paused at a ρ-dependent termination site. The coupling of transcription termination to translation blockage promotes termination of transcription when protein synthesis is interrupted. However, ρ-dependent termination is not very efficient, and transcription continues through a ρ-sensitive pause site as much as 50% of the time. ρ-dependent termination not only occurs at the ends of transcribed regions, but also accounts for ρ-dependent polarity (see “Polar Effects on Gene Expression” below).
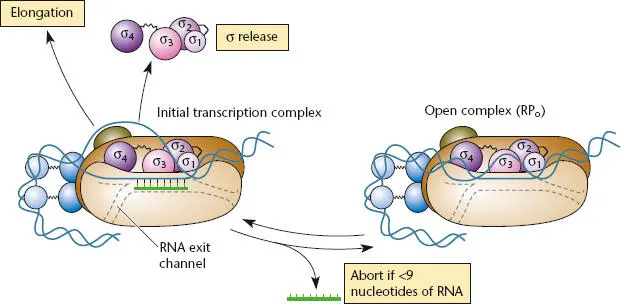
Figure 2.11 Abortive transcription and RNA polymerase escape from the promoter. RNA polymerase can escape from the promoter only if more than 10 or 11 nucleotides are polymerized. At 12 nucleotides, the RNA transcript displaces the σ 3.2region, which blocks the active-site channel. Abortive transcription results when short (<9 nucleotides) newly synthesized transcripts are released, and the complex returns to the open complex (RP o) state. With RNA polymerase escape, σ is released, and transcription elongation can continue.
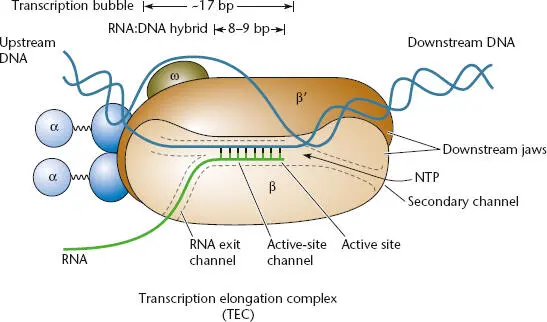
Figure 2.12 The transcription elongation complex (TEC). During elongation, nucleoside triphosphates (NTPs) enter through the secondary channel and are polymerized at the active site; the nascent RNA exits through the RNA exit channel. Modified from Geszvain K, Landick R, in Higgins NP (ed), The Bacterial Chromosome (ASM Press, Washington, DC, 2005).
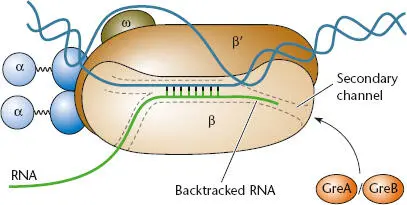
Figure 2.13 Backtracked transcription elongation complex (TEC). Backward movement of RNA polymerase results in placement of the 3′ end of the nascent RNA within the secondary channel, which prevents entry of nucleoside triphosphate (NTP) substrates. GreA and GreB can enter the secondary channel to cleave the nascent RNA, which repositions the 3′ end of the transcript in the active site, allowing transcription elongation to continue.
Transcription of the genes for all RNAs in the cell follows the same basic process. However, rRNAs and tRNAs play special roles in protein synthesis, so their fates after transcription differ from that of mRNAs.
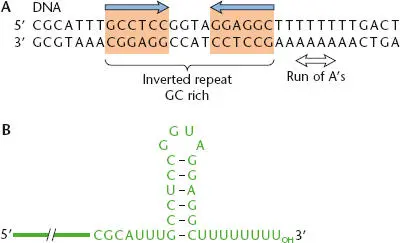
Figure 2.14 Transcription termination at a factor-independent termination site. (A)DNA sequence of a typical terminator site. (B)Sequence and structure of the RNA hairpin that forms in the nascent RNA as it emerges from RNA polymerase, which (in combination with the weak RNA-DNA hybrid) causes RNA polymerase to dissociate from the template DNA and release the RNA product.
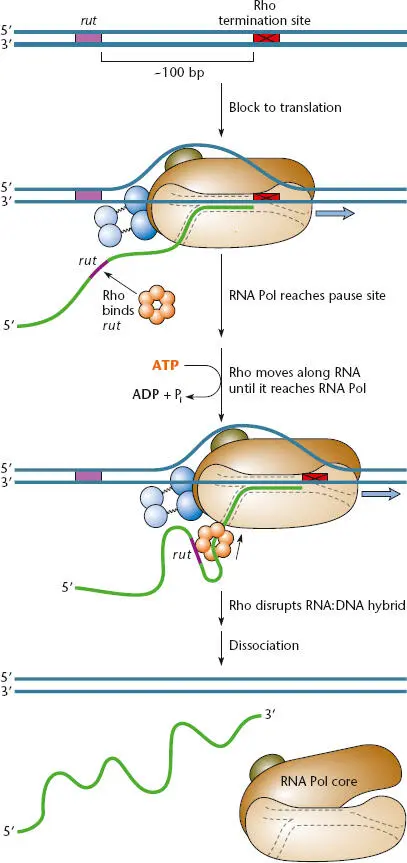
Figure 2.15 Model for factor-dependent transcription termination at a ρ-sensitive pause site. The ρ factor attaches to the RNA at a rut site if the RNA is not being translated (for example, if the ribosome has stopped at a termination codon) and forms a hexameric ring around the RNA. It then moves along the RNA with the cleavage of ATP until it catches up with RNA polymerase paused at a ρ-sensitive pause site. The helicase activity of the ρ factor then dissociates the RNA-DNA hybrid in the transcription bubble, causing the RNA polymerase and the RNA to be released.
The ribosomes are some of the largest structures in bacterial cells and are composed of both proteins and RNA. Bacterial ribosomes contain three types of rRNA: 16S, 23S, and 5S. The S value (from Svedberg, the name of the person who pioneered this way of measuring the sizes of molecules) is a measure of how fast a molecule sediments in an ultracentrifuge. In general, the higher the S value, the larger the RNA. The designation has persisted, even though this method of measuring molecular size is rarely used.
The rRNAs are among the most highly evolutionarily conserved of all the cellular constituents, as indeed are many of the components of the translational machinery. For this reason, they have formed the basis for molecular phylogeny ( Box 2.2). Comparisons of the sequences of rRNAs and other constituents of the translation apparatus from different species permit estimates to be made of how long ago these constituents separated evolutionarily.
In addition to their structural role in the ribosome, the rRNAs play a direct role in translation. The 23S rRNA is the peptidyltransferase enzyme, which joins amino acids into protein on the ribosome. The 23S rRNA therefore acts as a ribozyme, an RNA enzyme (see below). The 16S RNA lacks enzymatic activity but plays crucial roles in initiation and termination of translation, as well as in decoding of the sequence of the mRNA.
The rRNAs and tRNAs make up the bulk of the RNA in cells because of their central role in protein synthesis. In a rapidly growing bacterial cell, much of the total RNA synthesis is devoted to making these RNAs. Also, the rRNAs and tRNAs are far more resistant to degradation than mRNA. With this combination of a high synthesis rate and high stability, the rRNAs and tRNAs together can amount to more than 95% of the total RNA in a rapidly growing bacterial cell.
Not only do the rRNAs physically associate in the ribosome, but they also are synthesized together as long precursor RNAs containing all three forms of rRNA separated by so-called spacer regions. The precursors often contain one or more tRNAs, as well ( Figure 2.16), while other tRNAs are encoded in operons that do not include rRNA sequences. The individual rRNAs and tRNAs are released from the precursor RNAs by ribonucleases (RNases). Some of these RNases participate in both rRNA and tRNA processing and RNA degradation, while others are dedicated to a single function; for example, RNase P generates the precise 5′ end of tRNAs. At some point during the processing, individual nucleotides within the rRNA and tRNA precursor molecules are also modified to make the mature rRNAs and tRNAs.
Читать дальше
















