Jane Flint - Principles of Virology
Здесь есть возможность читать онлайн «Jane Flint - Principles of Virology» — ознакомительный отрывок электронной книги совершенно бесплатно, а после прочтения отрывка купить полную версию. В некоторых случаях можно слушать аудио, скачать через торрент в формате fb2 и присутствует краткое содержание. Жанр: unrecognised, на английском языке. Описание произведения, (предисловие) а так же отзывы посетителей доступны на портале библиотеки ЛибКат.
- Название:Principles of Virology
- Автор:
- Жанр:
- Год:неизвестен
- ISBN:нет данных
- Рейтинг книги:3 / 5. Голосов: 1
-
Избранное:Добавить в избранное
- Отзывы:
-
Ваша оценка:
- 60
- 1
- 2
- 3
- 4
- 5
Principles of Virology: краткое содержание, описание и аннотация
Предлагаем к чтению аннотацию, описание, краткое содержание или предисловие (зависит от того, что написал сам автор книги «Principles of Virology»). Если вы не нашли необходимую информацию о книге — напишите в комментариях, мы постараемся отыскать её.
Volume I: Molecular Biology
Volume II: Pathogenesis and Control
Principles of Virology, Fifth Edition
Principles of Virology — читать онлайн ознакомительный отрывок
Ниже представлен текст книги, разбитый по страницам. Система сохранения места последней прочитанной страницы, позволяет с удобством читать онлайн бесплатно книгу «Principles of Virology», без необходимости каждый раз заново искать на чём Вы остановились. Поставьте закладку, и сможете в любой момент перейти на страницу, на которой закончили чтение.
Интервал:
Закладка:
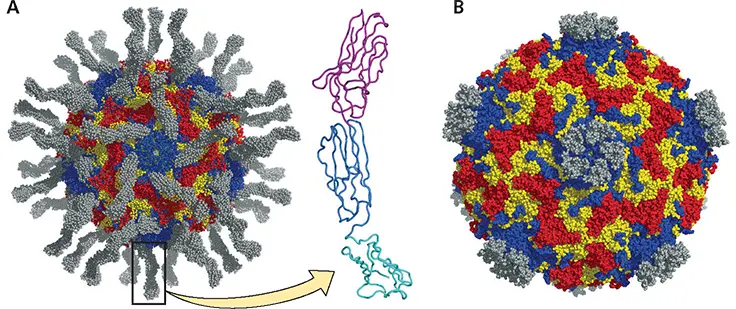
Figure 5.4 Picornavirus-receptor interactions. (A)Structure of poliovirus bound to a soluble form of CD155 (gray), derived by cryo-electron microscopy and image reconstruction. Capsid proteins are color coded (VP1, blue; VP2, yellow; VP3, red). The structure of a CD155 molecule (PDB ID: 1DGI) is shown at the right, with each Ig-like domain in a different color. The first Ig-like domain of CD155 (magenta) binds in the canyon of the viral capsid. (B)Structure of human rhinovirus type 2 bound to a soluble form of low-density lipoprotein receptor (gray). The receptor binds on the plateau at the 5-fold axis of symmetry of the capsid.
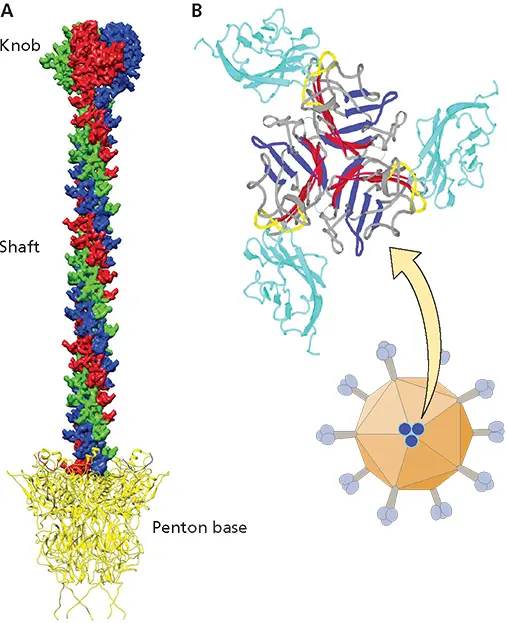
Figure 5.5 Structure of the adenovirus 12 knob bound to the CAR receptor. (A)Structure of a fiber protein, with knob, shaft, and tail domains labeled. Figure provided by Hong Zhou, University of California, Los Angeles, and Hongrong Liu, Hunan Normal University. (B)Ribbon diagram of the knob-CAR complex as viewed down the axis of the viral fiber. The trimeric knob is in the center (red-blue). The AB loop (yellow) of the knob protein contacts the first Ig-like domains of CAR molecules (cyan). The binding sites of both molecules require trimer formation.
Glycolipid receptors for polyomaviruses.The family Polyomaviridae includes simian virus 40, mouse polyomavirus, and human polyomavirus 1. Members of this family are unusual because they bind to gangliosides, which are glycosphingolipids with one or more sialic acids linked to a sugar chain. There are more than 40 known gangliosides, which differ in the position and number of sialic acid residues critical for virus binding. Simian virus 40, polyomavirus, and human polyomavirus 1 bind to three different types of ganglioside. Structural studies have revealed that sialic acid linked to galactose by an α(2,3) linkage binds to a pocket on the surface of the polyomavirus capsid. Gangliosides are highly concentrated in lipid rafts ( Chapter 2, Box. 2.1) and participate in signal transduction, two properties that are important during polyomavirus entry into cells.
Gangliosides are the only surface molecule identified, to date, to be required for human polyomavirus 1 entry. In contrast, after binding a ganglioside, mouse polyomavirus interacts with α 4β 1integrin to allow virus entry. Furthermore, gangliosides are not the primary receptor for simian virus 40. This virus first binds to major histocompatibility class I (MHC-I) molecules on the cell surface and the complex migrates to caveolin-rich membrane domains that are also enriched in gangliosides and mediate endocytosis ( Fig. 5.6). In contrast, another polyomavirus, human polyomavirus 2, relies not on gangliosides and caveolin pitmediated endocytosis but on the serotonergic receptor 5HT 2AR and ligand-induced clathrin-mediated endocytosis.
Alternative Attachment Strategies
The examples provided above highlight the diversity of cell surface molecules that can serve as viral receptors and demonstrate that entry of many viruses requires more than one cell surface molecule. In contrast, some nonenveloped virus particles bind to different cell receptors, depending on the nature of the virus isolate or the cell line. Often passage of viruses in cells in culture selects variants that bind heparan sulfate. Infection of cells with foot-and-mouth disease virus type A12 requires integrin α vβ 3. However, the receptor for the O strain of this virus, which has been extensively passaged in cells in culture, is not integrin α vβ 3but cell surface heparan sulfate. On the other hand, the type A12 strain cannot infect cells that lack integrin α vβ 3, even if heparan sulfate is present.
Transmembrane Glycoproteins of Enveloped Viruses Mediate Attachment and Entry
The lipid membranes of enveloped viruses originate from those of the host cells. Membrane-spanning viral proteins are inserted into these cellular membranes by the same mechanisms as cellular integral membrane proteins and incorporated in the budding virus particles. Attachment sites on one or more of these envelope proteins bind to specific receptors. The two best-studied examples of enveloped virus attachment and its consequences are provided by the interactions of the envelope proteins of influenza A virus and the human immunodeficiency virus type 1 with their receptors.
Influenza virus.The family Orthomyxoviridae comprises the three genera of influenza viruses, A, B, and C. These viruses bind to negatively charged, terminal sialic acid moieties present in oligosaccharide chains that are covalently attached to cell surface glycoproteins or glycolipids. The presence of sialic acid on most cell surfaces accounts for the ability of influenza virus particles to attach to many types of cells. The interaction of influenza virus with individual sialic acid moieties is of low affinity. However, the opportunity for multiple interactions among the numerous attachment proteins on the surface of the virus particle and multiple sialic acid residues on cellular glycoproteins and glycolipids results in a high overall avidity of the virus particle for the cell surface.
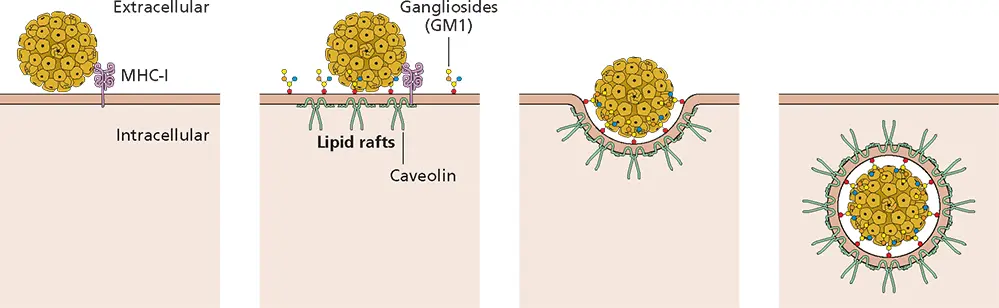
Figure 5.6 Entry of polyomavirus simian virus 40. Simian virus 40 interacts first with MHC class I molecules and bound virus particles move into small pits, 60 to 80 nm in diameter, rich in caveolin, known as caveolae. These pits are enriched in gangliosides and cholesterol. Several polyomaviruses bind to gangliosides with a terminal sialic acid linked by an α(2,3) bond to the penultimate galactose. Binding of virus particles to gangliosides induces intracellular signaling and membrane curvature that result in formation of caveolae that are endocytosed, bringing the virus particles inside the cell.
The virus glycoprotein that binds to the cell receptor sialic acid is HA (hemagglutinin). The HA trimer is synthesized as a precursor that is glycosylated, and subsequently each monomer is cleaved to form HA1 and HA2 subunits that remain attached via disulfide bonds. Each HA monomer consists of a long, helical stalk anchored in the membrane by HA2 and topped by a large HA1 globule, which includes the sialic acid-binding pocket ( Fig. 5.7A). While attachment of all influenza A virus strains requires sialic acid, strains vary in their affinities for different sialyloligosaccharides. For example, human virus strains bind preferentially sialic acids attached to galactose via an α(2,6) linkage, the major sialic acid present on human respiratory epithelium ( Fig. 5.7B). Avian virus strains bind preferentially to sialic acids attached to galactose via an α(2,3) linkage, the major sialic acid in the duck gut epithelium. Amino acids in the sialic acid-binding pocket of HA ( Fig. 5.7A, inset) determine which sialic acid is preferred and can therefore influence viral host range. It is thought that an amino acid change in the sialic acid-binding pocket of the 1918 influenza virus, which may have evolved from an avian virus, allowed it to recognize the α(2,6)-linked sialic acids that predominate in human cells.
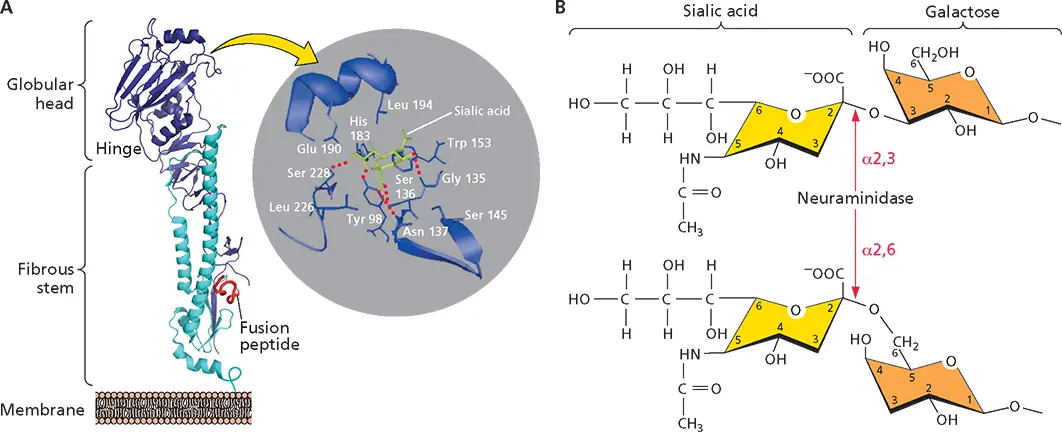
Figure 5.7 Interaction of sialic acid receptors with the hemagglutinin of influenza viruses. (A)Structure of the cleaved ectodomain of a monomer of the HA1-HA2 glycoprotein (based on X-ray crystallography data; PDB ID: 4FNK). HA1 (blue) and HA2 (cyan) subunits are held together by a disulfide bridge as well as by many noncovalent interactions. The fusion peptide at the N terminus of HA2 is indicated (red). (Inset) Close-up of the receptor-binding site with a bound sialic acid molecule. Side chains of the conserved amino acids that form the site and hydrogen-bond with the receptor are included. (B)The structure of a terminal sialic acid moiety that is recognized by HA. Sialic acid is attached to galactose by an α(2,3) (top) or an α(2,6) (bottom) linkage. The site of cleavage by the influenza virus envelope glycoprotein neuraminidase is indicated. The sialic acid shown is N -acetylneuraminic acid, which is the preferred receptor for influenza A and B viruses.
Читать дальшеИнтервал:
Закладка:
Похожие книги на «Principles of Virology»
Представляем Вашему вниманию похожие книги на «Principles of Virology» списком для выбора. Мы отобрали схожую по названию и смыслу литературу в надежде предоставить читателям больше вариантов отыскать новые, интересные, ещё непрочитанные произведения.
Обсуждение, отзывы о книге «Principles of Virology» и просто собственные мнения читателей. Оставьте ваши комментарии, напишите, что Вы думаете о произведении, его смысле или главных героях. Укажите что конкретно понравилось, а что нет, и почему Вы так считаете.











