Jane Flint - Principles of Virology
Здесь есть возможность читать онлайн «Jane Flint - Principles of Virology» — ознакомительный отрывок электронной книги совершенно бесплатно, а после прочтения отрывка купить полную версию. В некоторых случаях можно слушать аудио, скачать через торрент в формате fb2 и присутствует краткое содержание. Жанр: unrecognised, на английском языке. Описание произведения, (предисловие) а так же отзывы посетителей доступны на портале библиотеки ЛибКат.
- Название:Principles of Virology
- Автор:
- Жанр:
- Год:неизвестен
- ISBN:нет данных
- Рейтинг книги:3 / 5. Голосов: 1
-
Избранное:Добавить в избранное
- Отзывы:
-
Ваша оценка:
- 60
- 1
- 2
- 3
- 4
- 5
Principles of Virology: краткое содержание, описание и аннотация
Предлагаем к чтению аннотацию, описание, краткое содержание или предисловие (зависит от того, что написал сам автор книги «Principles of Virology»). Если вы не нашли необходимую информацию о книге — напишите в комментариях, мы постараемся отыскать её.
Volume I: Molecular Biology
Volume II: Pathogenesis and Control
Principles of Virology, Fifth Edition
Principles of Virology — читать онлайн ознакомительный отрывок
Ниже представлен текст книги, разбитый по страницам. Система сохранения места последней прочитанной страницы, позволяет с удобством читать онлайн бесплатно книгу «Principles of Virology», без необходимости каждый раз заново искать на чём Вы остановились. Поставьте закладку, и сможете в любой момент перейти на страницу, на которой закончили чтение.
Интервал:
Закладка:
Receptor genes have also been used to produce transgenic mice that synthesize receptor proteins. Such transgenic animals can serve as useful models in the study of human viral diseases. For example, mouse cells are permissive for poliovirus reproduction, and susceptibility is limited onlyby the absence of the virus receptor. Consequently, it was possible to develop a small-animal model for poliomyelitis by producing transgenic mice that synthesize this receptor. Inoculation of these transgenic mice with poliovirus by various routes produces paralysis, as is observed in human poliomyelitis. These mice were the first new animal model created by transgenic technology for the study of viral disease. Similar approaches have subsequently led to animal models for diseases caused by measles virus and echoviruses.
Virus-Receptor Interactions
Animal viruses can have multiple receptor-binding sites on their surfaces. Receptor-binding sites for enveloped viruses are usually provided by oligomeric integral membrane glycoproteins that have been incorporated into the cell-derived membranes of virus particles. For nonenveloped viruses, this function is usually provided by one or more of the viral capsid proteins. Typically, these form projections from or indentations in the virus particle surface. The general mechanisms of virus-receptor interactions are illustrated by the best-studied examples described below.
Nonenveloped Virus Receptor Binding
Attachment via surface features: canyons and loops.Members of the enterovirus genus of the Picornaviridae include human polioviruses, Coxsackieviruses, echoviruses, enteroviruses, and rhinoviruses. The receptor for poliovirus, CD155, was identified by using a DNA transformation and cloning strategy ( Fig. 5.2). Transfection of poliovirus RNA into mouse cells in culture was shown to lead to poliovirus reproduction, indicating that there is no intracellular block to virus multiplication. Subsequently, human DNA was introduced into mouse cells and screened for the ability to confer susceptibility to poliovirus infection. The human gene recovered from susceptible mouse cells proved to encode CD155, a glycoprotein that is a member of the immunoglobulin (Ig) superfamily ( Fig. 5.3).
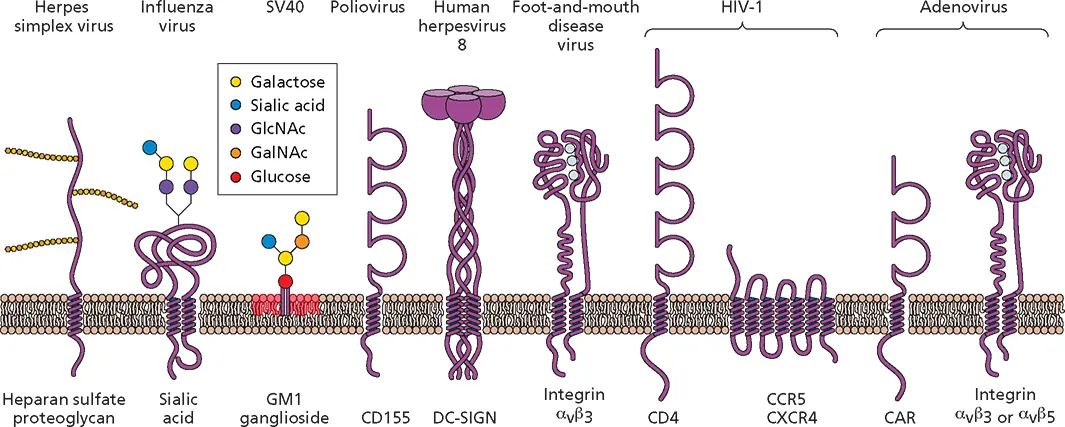
Figure 5.3 Some receptors for virus particles. Schematic diagrams of cell molecules that function during virus entry. Note that CD155, CD4, and CAR are all members of the Ig superfamily. CAR, Coxsackievirus and adenovirus receptor; CCR5, chemokine receptor type 5; CD, cluster of differentiation; CXCR4, chemokine receptor type 4; DC-SIGN, dendritic cell-specific intercellular adhesion molecule 3-grabbing non-integrin; GalNAc, N -acetylgalactosamine; GlcNAc, N -acetylglucosamine; GM, monosialotetrahexosyl. Integrins are bound to divalent Ca 2+ions (light blue balls).
Different proteins serve as receptors for other members of the genus. More than 150 rhinovirus genotypes have been identified and classified on the basis of genome sequence into three species, A, B, and C. The cell surface receptor bound by most A and B species of rhinoviruses was identified using a monoclonal antibody that blocks rhinovirus infection and that recognizes a cell surface protein. This monoclonal antibody was used to isolate a 95-kDa cell surface glycoprotein by affinity chromatography. Amino acid sequence analysis of the purified protein, which bound to rhinovirus in vitro , identified it as ICAM-1 (integral membrane protein intercellular adhesion molecule 1, also known as CD54). ICAM-1 is not a universal receptor for all A and B species, as some members can bind the low-density lipoprotein receptor. Rhinovirus C species bind the cadherin-related family member 3.
The RNA genomes of picornaviruses are protected by capsids built from four virus-encoded proteins, VP1, VP2, VP3, and VP4, arranged with icosahedral symmetry (see Fig. 4.12). While the capsids of rhinoviruses and polioviruses have deep canyons surrounding the 12 5-fold axes of symmetry ( Fig. 5.4), cardioviruses and aphthoviruses lack this feature. The canyons in the capsids of some rhinoviruses and enteroviruses are the sites of interaction with cell surface receptors. Amino acids that line the canyons are more highly conserved than any others on the surface of virus particles, and their substitution can alter the binding affinity to cells. Poliovirus bound to a receptor fragment comprising CD155 domains 1 and 2 has been visualized in reconstructed images from cryo-electron microscopy. The results indicate that the first domain of CD155 binds to the central portion of the canyon in an orientation oblique to the surface of the virus particle ( Fig. 5.4A).
Canyons are present in the capsid of rhinovirus type 2, but they are not the binding sites for the receptor, low-density lipoprotein receptor. Rather, this site on the capsid is located on the star-shaped plateau at the 5-fold axis of symmetry ( Fig. 5.4B). Sequence and structural comparisons have revealed why different rhinovirus serotypes bind distinct receptors. A critical lysine residue in VP1 interacts with a negatively charged region of the low-density lipoprotein receptor and is conserved in all rhinoviruses that bind this receptor. This lysine is not found in VP1 of rhinoviruses that bind ICAM-1.
For picornaviruses with capsids that do not have prominent canyons, including group A Coxsackieviruses and foot-and-mouth disease virus, attachment is mediated by VP1 surface loops that include amino acid sequence motifs recognized by their integrin receptors.
Attachment via protruding fibers.The results of competition experiments indicated that members of two different virus families, group B Coxsackieviruses and many human adenoviruses, share a cell receptor. This receptor is a 46-kDa member of the Ig superfamily named CAR for Coxsackievirus and adenovirus receptor ( Fig. 5.3). Binding to this receptor is not sufficient for infection by most adenoviruses. Interaction with a coreceptor, the α vintegrin α vβ 3or α vβ 5, is required for uptake of the capsid into the cell by receptor-mediated endo-cytosis. An exception is adenovirus type 9, which can infect hematopoietic cells after binding directly to α vintegrins. Some adenoviruses of subgroup B bind CD46, which is also a cell receptor for some strains of measles virus, an enveloped member of the Paramyxoviridae .
The nonenveloped DNA-containing adenoviruses are much larger than picornaviruses, and their icosahedral capsids are more complex, comprising at least 10 different proteins. Electron microscopy shows that fibers protrude from each adenovirus penton ( Fig. 5.5). The fibers are composed of homotrimers of the adenovirus fiber protein and are anchored in the pentameric penton base; both proteins have roles to play in virus attachment and uptake.
For many adenovirus serotypes, attachment via the fibers is necessary but not sufficient for infection. A region comprising the N-terminal 40 amino acids of each subunit of the fiber protein is bound noncovalently to the penton base ( Fig. 5.5A). The central shaft is composed of repeating motifs of approximately 15 amino acids; the length of the shaft in different serotypes is determined by the number of these repeats. The three constituent shaft regions appear to form a rigid triple-helical structure in the trimeric fiber. The C-terminal 180 amino acids of each subunit interact to form a terminal knob. Genetic analyses and competition experiments indicate that determinants for the initial, specific attachment to host cell receptors reside in this knob. The structure of this domain bound to CAR reveals that surface loops of the knob contact one face of the receptor ( Fig. 5.5B). Attachment to integrins is mediated by amino acid sequences in each of the five subunits of the adenovirus penton base that mimic the normal ligands of these molecules.
Читать дальшеИнтервал:
Закладка:
Похожие книги на «Principles of Virology»
Представляем Вашему вниманию похожие книги на «Principles of Virology» списком для выбора. Мы отобрали схожую по названию и смыслу литературу в надежде предоставить читателям больше вариантов отыскать новые, интересные, ещё непрочитанные произведения.
Обсуждение, отзывы о книге «Principles of Virology» и просто собственные мнения читателей. Оставьте ваши комментарии, напишите, что Вы думаете о произведении, его смысле или главных героях. Укажите что конкретно понравилось, а что нет, и почему Вы так считаете.











