Jane Flint - Principles of Virology
Здесь есть возможность читать онлайн «Jane Flint - Principles of Virology» — ознакомительный отрывок электронной книги совершенно бесплатно, а после прочтения отрывка купить полную версию. В некоторых случаях можно слушать аудио, скачать через торрент в формате fb2 и присутствует краткое содержание. Жанр: unrecognised, на английском языке. Описание произведения, (предисловие) а так же отзывы посетителей доступны на портале библиотеки ЛибКат.
- Название:Principles of Virology
- Автор:
- Жанр:
- Год:неизвестен
- ISBN:нет данных
- Рейтинг книги:3 / 5. Голосов: 1
-
Избранное:Добавить в избранное
- Отзывы:
-
Ваша оценка:
- 60
- 1
- 2
- 3
- 4
- 5
Principles of Virology: краткое содержание, описание и аннотация
Предлагаем к чтению аннотацию, описание, краткое содержание или предисловие (зависит от того, что написал сам автор книги «Principles of Virology»). Если вы не нашли необходимую информацию о книге — напишите в комментариях, мы постараемся отыскать её.
Volume I: Molecular Biology
Volume II: Pathogenesis and Control
Principles of Virology, Fifth Edition
Principles of Virology — читать онлайн ознакомительный отрывок
Ниже представлен текст книги, разбитый по страницам. Система сохранения места последней прочитанной страницы, позволяет с удобством читать онлайн бесплатно книгу «Principles of Virology», без необходимости каждый раз заново искать на чём Вы остановились. Поставьте закладку, и сможете в любой момент перейти на страницу, на которой закончили чтение.
Интервал:
Закладка:
Packaging by Specialized Viral Proteins
In many virus particles, the genome is associated with specialized nucleic acid-binding proteins, such as the nucleocapsid proteins of (−) strand RNA viruses and (+) strand retroviruses, or the core proteins of adenoviruses. An important function of such proteins is to condense and protect viral genomes. Consequently, they do not recognize specific nucleic acid sequences but rather bind nonspecifically to RNA or DNA genomes. This mode of binding is exemplified by the structure of the vesicular stomatitis virus N protein, in which 9 nucleotides of RNA are tightly but nonspecifically bound in a cavity formed between the two domains of each N protein molecule ( Fig. 4.7). These protein-RNA interactions both sequester the RNA genome and organize it into a helical structure. Formation of helical ribonucleoproteins by two-domain RNA-binding proteins is a packaging mechanism common among (−) strand RNA viruses in the order Mononegavirales : the N proteins of representatives of other families in the order exhibit the same two-lobed structure and mode of RNA binding ( Fig. 4.21).
Electron microscopy of cores released from adenovirus particles and cryo-EM of virus particles have suggested that the internal nucleoprotein is also arranged in some regular fashion. However, how the viral DNA genome is organized and condensed by the core proteins is not known: the nucleoprotein was not observed in the high-resolution structures of adenovirus particles described previously, and the structures of core proteins have not been determined. The fundamental DNA packaging unit is a multimer of protein VII, which appears as beads on a string of adenoviral DNA when other core proteins are removed. Protein VII binds tightly to and condenses double-stranded DNA in vitro , consistent with a packaging function, but has been reported to be dispensable for assembly of virus particles ( Chapter 13). Protein VII and the other core proteins are basic, as would be expected for proteins that bind to a negatively charged DNA molecule without sequence specificity.
Packaging by Cellular Proteins
The final mechanism for condensing the viral genome, by cellular proteins, is unique to polyomaviruses, such as simian virus 40, and papillomaviruses. The circular, double-stranded DNA genomes released from these virus particles are organized into nucleosomes that contain the four cellular core histones, H2A, H2B, H3, and H4, to form a minichromosome. Comparison of cryo-EM structures of purified particles of the human polyomavirus BK virus and virus-like particles formed only from VP1 (the major structural protein) has revealed two radial shells of the DNA genome within virus particles ( Fig. 4.20B). The thickness of these shells (24 Å) and the distance between them match those of double-stranded DNA within a human nucleosome. The 20 or so nucleosomes that are associated with polyomaviral genomes condense the DNA by a factor of ~7. This packaging mechanism is elegant, with two major advantages: none of the limited viral genetic information needs to be devoted to DNA-binding proteins, and the viral genome, which is transcribed by cellular RNA polymerase II, enters the infected cell nucleus as a nucleoprotein closely resembling the cellular templates for this enzyme.
BOX 4.7
EXPERIMENTS
A high-resolution view of an encapsidated viral genome
The small icosahedral capsid ( T = 3) of the Escherichia coli bacteriophage MS2 is built from dimers of a single coat protein and one copy of a maturation protein, which is responsible for delivery of the genome to host cells. The capsid contains a single-stranded (+) RNA genome of 3,569 bases, the first genome to be sequenced completely. Cryo-EM of MS2 particles and averaging of more than 300,000 images withoutimposing any symmetry allowed visualization of >80% of the viral RNA genome at 6-Å resolution. As shown in panel A of the figure, most of the RNA showed prominent major and minor grooves; i.e., it is double-stranded, and is organized as stem-loops. Some of these structures could be examined at higher resolution (3.6 Å), indicating that they are more ordered, and their sequences determined from features of purines and pyrimidines seen in the EM density (panel B in the figure). This study illustrates the power of asymmetric reconstruction of images collected by cryo-EM, and the high degree of viral genome folding that can be imposed by interactions with capsid proteins.
Dai X, Li Z, Lai M, Shu S, Du Y, Zhou ZH, Sun R. 2017. In situ structures of the genome and genome-delivery apparatus in a single-stranded RNA virus. Nature 541:112–116.
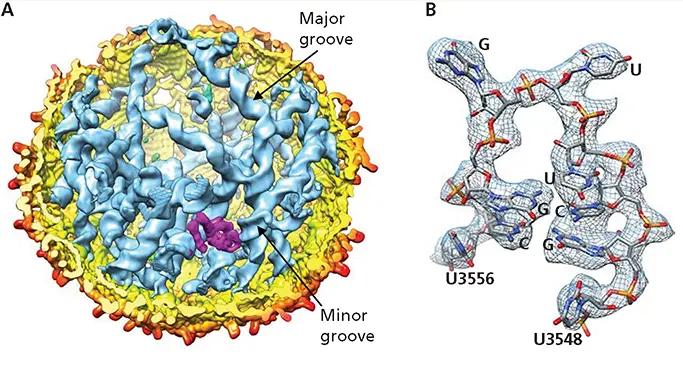
Structure of a small viral RNA genome. (A)Cut-open view of the asymmetric reconstruction of MS2 (6 Å) with the capsid shell colored yellow to red (by radial distance), the maturation protein in magenta, and the RNA in blue with major and minor grooves indicated. (B)A segment of an RNA stem-loop observed at 3.6-Å resolution, with RNA backbone and bases imposed on the EM density (mesh). Adapted from Dai X et al. 2017. Nature 541:112–116, with permission. Courtesy of H. Zhou, University of California, Los Angeles. See also https://media.nature.com/original/nature-assets/nature/journal/v541/n7635/extref/nature20589-sv1.mp4.
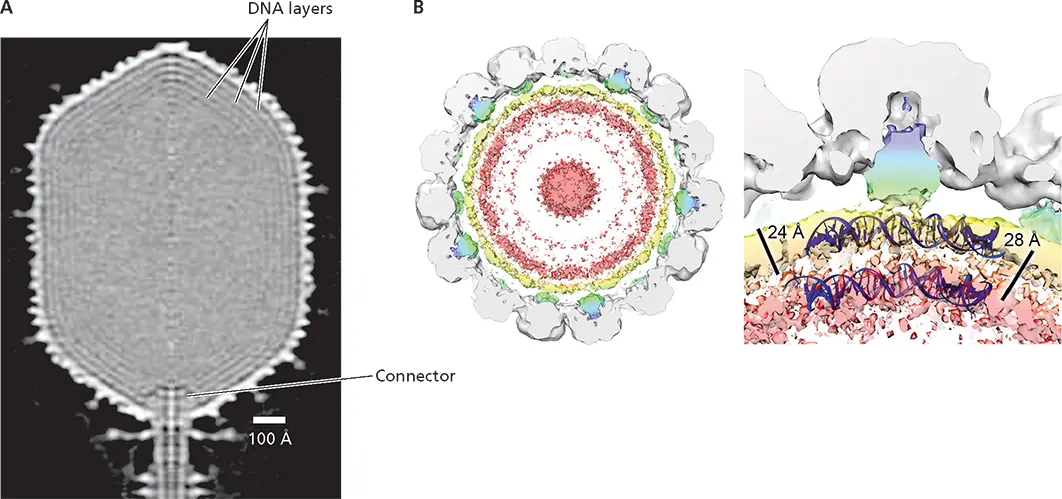
Figure 4.20 Packing of double-stranded DNA genome. (A)Dense packing in the head of bacteriophage T4 DNA. The central section of a 22-Å cryo-EM reconstruction of the head of bacteriophage T4 viewed perpendicular to the fivefold axis is shown. The concentric layers seen underneath the capsid shell have been attributed to the viral DNA genome. The connector, which is derived from the portal structure by which the DNA genome enters the head during assembly, connects the head to the tail. Adapted from Fokine A et al. 2004. Proc Natl Acad Sci U S A 101:6003–6008, with permission. Courtesy of M. Rossmann, Purdue University. (B)(Left) Cryo-EM reconstruction of the polyomavirus BK virus shown as a 40-Å-thick slab, with fitted VP1 density in gray, density assigned to the minor structural proteins VP2 and VP3 in blue/green, and to packaged double-stranded DNA in yellow to pink. These colored densities were not observed in virus-like particles assembled from only VP1. (Right) An enlarged view of the density below a single VP1 penton, indicating the thickness of the radial layers of DNA and the spacing between them. A model of double-stranded DNA as it appears when wrapped on a human histone (blue) is superimposed. Adapted from Hurdiss DL et al. 2016. Structure 24:528–536, licensed under CC BY 4.0. Courtesy of N.A. Ranson, University of Leeds, United Kingdom.
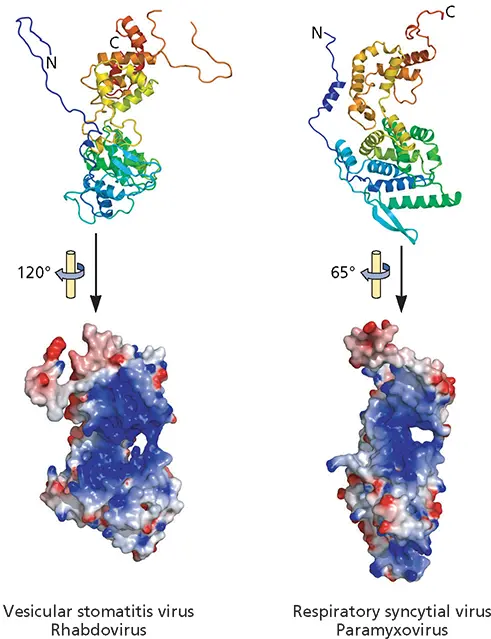
Figure 4.21 Conserved organization of the RNA-packaging proteins of nonsegmented (−) strand RNA viruses. Ribbon diagrams of the N proteins indicated are shown at the top, colored from purple at the N terminus to red at the C terminus. Their electrostatic surfaces from negative (red) to positive (blue) are shown in the space-filling models below, with the molecules rotated as indicated to show the RNA-binding cleft (blue) most clearly. Although differing in structural details, these N proteins share a two-lobed structure (top) and an RNA-binding cleft between the two lobes. Adapted from Ruigrok RW et al. 2011. Curr Opin Microbiol 14:504–510, with permission. Courtesy of D. Kolakofsky, University of Geneva.
Читать дальшеИнтервал:
Закладка:
Похожие книги на «Principles of Virology»
Представляем Вашему вниманию похожие книги на «Principles of Virology» списком для выбора. Мы отобрали схожую по названию и смыслу литературу в надежде предоставить читателям больше вариантов отыскать новые, интересные, ещё непрочитанные произведения.
Обсуждение, отзывы о книге «Principles of Virology» и просто собственные мнения читателей. Оставьте ваши комментарии, напишите, что Вы думаете о произведении, его смысле или главных героях. Укажите что конкретно понравилось, а что нет, и почему Вы так считаете.











