For a given substituent (EWG or EDG), the extent of its electronic effects on the side group (–COO −) of XC 6H 4COO −is different when the substituent is placed on the para ‐position and on the meta ‐position. Therefore, the σ constants for the substituent on para ‐position (σ para) and on meta ‐position (σ meta) are different. For example, the electron withdrawing effects of –NO 2on both its para ‐ and meta ‐carbons are mainly due to its conjugation effect. The conjugation effect of a para –NO 2to the side group –COO −is stronger than a meta –NO 2. Therefore, the σ para(0.81) is greater than the σ meta(0.71) for –NO 2(σ > 0 for EWGs) [1]. This is also true for some other EWGs (–CN, –CF 3, and –CO 2Me) whose major electronic effects on both their para ‐ and meta ‐carbons are conjugation effects, and we have σ para= 0.70 and σ meta= 0.62 for –CN; σ para= 0.53 and σ meta= 0.46 for –CF 3; and σ para= 0.44 and σ meta= 0.35 for –CO 2Me [1]. For EDGs whose major electronic effects are conjugation effects, the absolute value of σ parais greater than the absolute vale of σ meta(σ < 0 for EDGs). For example, we have σ para= –0.14 and σ meta= –0.06 for –CH 3; and σ para= –0.32 and σ meta= –0.10 for –N(CH 3) 2[1].
For various aromatic compounds (substituted benzenes), the substituents have substantial influence on rates and equilibrium for their reactions occurring on the phenyl ring as well as on the side group attached to the ring ( Eq. 1.57):
(1.57) 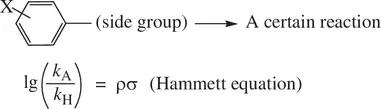
In Equation 1.57, the chemical structure shows a general substituted benzene with –X being an EWG or EDG at the para ‐position or meta ‐position to the side group. A reaction may occur on the side group (typically on the first atom of the group connecting to the ring) with the aromatic ring intact. A reaction may also occur on the ring with the side group intact. k Aand k Hare the rate constants for a certain reaction of the compound with and without the substituent, respectively. lg is the common logarithm (10‐based logarithm). The σ value is the Hammett substituent constant of –X as defined in Equation 1.56. It has been found that for various reactions occurring on the phenyl ring and on the side group, as the substituent (–X) is altered (σ changes accordingly), the plot of lg( k A/ k H) versus the substituent constant σ gives a straight line, showing that the lg( k A/ k H) value is directly proportional to σ of the substituent and is independent of its structure. The slope ( ρ ) for the lg( k A/ k H) versus σ plot is characteristic of different reactions. The quantitative relationship between lg( k A/ k H) and the substituent constant σ in Equation 1.57is referred to as the Hammett equation. For a given reaction that occurs on the phenyl ring or on the side group, ρ (rho) in the Hammett equation is a constant.
For the reactions which develop a positive charge (or destroy a negative charge) in the transition state of the rate‐determining step, ρ in the Hammett equation is a negative constant (ρ < 0). An EWG (σ > 0) will destabilize the transition state carrying a positive charge, making the k A/ k H< 1 [lg( k A/ k H) < 0]. An EDG (σ < 0) will stabilize the transition state carrying a positive charge, making the k A/ k H> 1 [lg( k A/ k H) > 0]. Both EWG and EDG require a negative ρ constant to maintain the Hammett equation. For the reactions which develop a negative charge (or destroy a positive charge) in the transition state of the rate‐determining step, ρ in the Hammett equation is a positive constant (ρ > 0). An EWG (σ > 0) will stabilize the transition state carrying a negative charge, making the k A/ k H> 1 [lg( k A/ k H) > 0]. An EDG (σ < 0) will destabilize the transition state carrying a negative charge, making the k A/ k H< 1 [lg( k A/ k H) < 0]. Both EWG and EDG require a positive ρconstant to maintain the Hammett equation.
When a reaction occurs on the aromatic ring to develop a positive or a negative charge in the transition state, the influence of a substituent (EWG or EDG) on the reaction rate is usually stronger than that on a reaction taking place in the side group. Therefore, the absolute value of the ρ constant for a reaction occurring on the aromatic ring is often greater than the absolute value of the ρ constant for a reaction occurring on the side group (exceptions exist). All this is illustrated by the reactions in Figure 1.6, with all the ρ constants taken from Ref. [1].
Figure 1.6a is an electrophilic aromatic substitution reaction (occurring on the aromatic ring) via an arenium cation (a positive charge is developed in the transition state). The substituent can strongly interact with the positive charge in the transition state formed in the ring, giving rise to a big negative ρ constant ( ρ = –12.1) for the reaction. Figure 1.6b is a nucleophilic aromatic substitution reaction (occurring on the aromatic ring) via an intermediate Meisenheimer anion (a negative charge is developed in the transition state). The substituent can strongly interact with the negative charge in the transition state, giving rise to a positive ρ constant ( ρ = 3.9) for the reaction. On the other hand, Figure 1.6c reaction takes place at the first atom of a side chain attaching to the aromatic ring and the ring is intact in the reaction. A carbocation is formed in the course of the reaction, thus a positive charge is developed in the transition state. The ρ constant for this reaction is negative ( ρ = –4.54). Its absolute value is much smaller than that of the ρ constant for Reaction (a) occurring in the ring. As the substituent is distanced from the reactive center, its electronic effect on the reaction is getting smaller. Figure 1.6d reaction occurs in the side group, with a negative charge in the phenoxide oxygen destroyed (equivalent to development of a positive charge). Therefore, the reaction has a small negative ρ constant ( ρ = –1.12). Its absolute value is substantially smaller than those of the ρ constants for Reactions (a) and (b), indicating weaker electronic effects of substituents on the side groups relative to the effects on the ring. Figure 1.6e reaction also occurs on the side group, with a positive charge in the nitrogen atom destroyed (equivalent to development of a negative charge) in the course of the reaction. For this reaction, the Hammett equation can be established using the acid dissociation constants of XC 6H 4NH 3 +, formulated as lg( K A/ K H) = ρ σ, where K Aand K Hare dissociation constants of XC 6H 4NH 3 +and C 6H 5NH 3 +, respectively. Since the reaction occurs on the side group and destroys a positive charge, it has a small positive ρ constant ( ρ = 2.77).
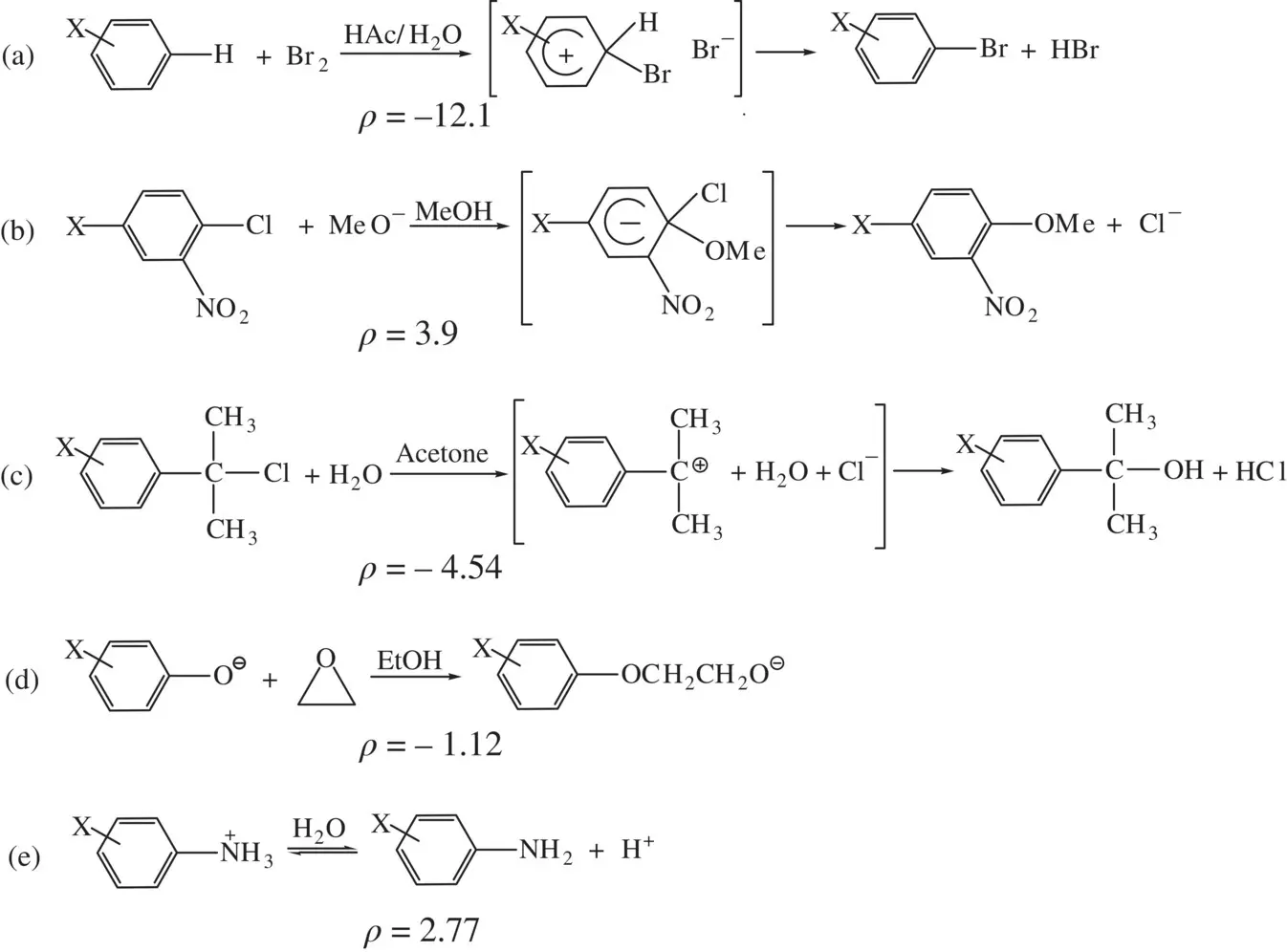
FIGURE 1.6 The ρ constants for various reactions of substituted benzenes. (a) Electrophilic aromatic substitution, (b) Nucleophilic aromatic substitution, (c) Hydrolysis, (d) S N2 reaction, and (e) Dissociation.
Читать дальше














