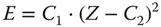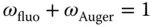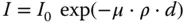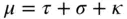1 ...7 8 9 11 12 13 ...24 (2.4) 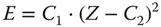
with
| E |
= |
energy difference between the electron levels |
| Z |
= |
atomic number of the atom |
| C 1, C 2 |
= |
constants |
This relation is shown for different lines as a function of the atomic number in Figure 2.2. Detailed information can be found in Tables A.4–A.9.
Figure 2.2shows that the energy of the characteristic radiation increases with the atomic number; it also shows that the line energies of an element decrease from the K series over the L series to the M series, and that in the energy range of approximately 1–40 keV, which is commonly used for X-ray spectrometry, almost all elements emit their characteristic radiation. Exceptions are only elements with very low atomic numbers ( Z ≤ 7).
2.2.2 Intensity of the Characteristic Radiation
The intensity of the characteristic radiation is determined by the number and type of the atoms in the excited volume; in other words, the intensity depends on their mass fraction w . It is further influenced by the intensity I 0of the primary radiation. The photo-absorption coefficient τ ( E ) describes the probability for the excitation of an atom by X-rays with energy E . If an inner electron shell is ionized, the transition from an outer electron shell is described by the transition probability p . For shells that are close together, p has the greatest value; therefore, the α-lines are the most intense within a series. For energy levels with a larger difference the transition probabilities are smaller, i.e. the intensities of the β-lines are correspondingly lower; however, their energies are higher. Special electron transitions can be excluded as a result of quantum selection rules; for these transitions, the transition probabilities are p = 0. For the main electron transitions, the labels of the corresponding X-ray lines are shown in Figure 2.3.
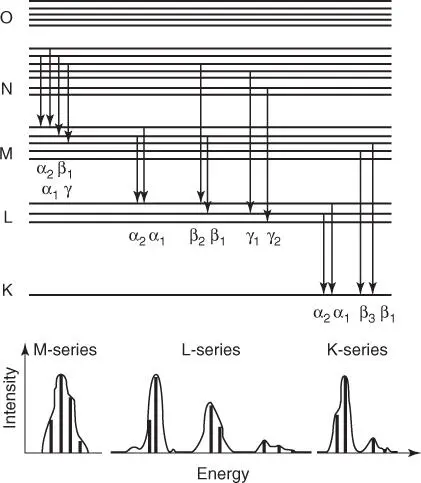
Figure 2.3 Electron transitions with the corresponding line names.
The approximate intensity ratios within a series as well as between the main lines of the different series (dependent on the excitation conditions) are shown in Table 2.1.
Table 2.1 Approximate relative line intensities of main X-ray lines.
| K-series |
L-series |
M-series |
| 100 |
Approx. 5–10 |
Approx. 1 |
| Kα 1 |
K–L3 |
100 |
Lα 1 |
L3–M5 |
100 |
Mα 1 |
M5–N7 |
100 |
| Kα 2 |
K–L2 |
50 |
Lβ 1 |
L2–M4 |
50 |
Mα 2 |
M5–N6 |
100 |
| Kα 1,2 |
K–L23 |
150 |
Lβ 2 |
L3–N5 |
12 |
Mβ |
M4–N6 |
52 |
| Kβ 1,3 |
K–M2,3 |
15 |
Lγ 1 |
L2–N4 |
6 |
Mγ |
M3–N5 |
5 |
| Kβ 2,4 |
K–N2,3 |
3 |
Ll |
L3–M1 |
5 |
|
|
|
|
|
|
Lβ 3 |
L1–M3 |
10 |
|
|
|
|
|
|
Lβ 4 |
L1–M2 |
7 |
|
|
|
|
|
|
Lη |
L2–M1 |
5 |
|
|
|
After an electron transition, the released energy is emitted. This is possible by the emission of characteristic radiation, which is due to the large energy differences of the involved electron levels in the range of X-ray radiation. Another possibility is the transfer of the energy to an outer electron of the atom under consideration and its emission. This process is called Auger effect. The probability for the emission of an X-ray photon again depends on the atomic number and is called fluorescence yield ω fluo. This probability is shown in Figure 2.4; more precise values for the fluorescence yield of the individual elements can be taken from Table A.11. Since an ionized atom always goes into the stable basic state,
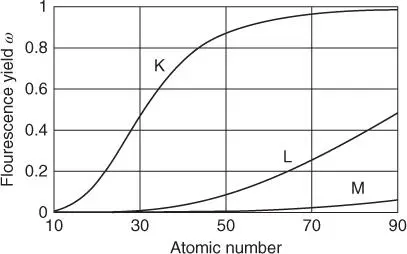
Figure 2.4 Fluorescence yield as a function of the atomic number.
The relation shows that the fluorescence yields are very small for elements with low atomic numbers. Most of the atoms then emit the energy released by the transition to the ground state as Auger electron and only very few as X-ray photons. This is the main reason for the low sensitivity of X-ray spectrometry for light elements. For higher radiation energies, the fluorescence yield increases significantly.
2.2.3 Nomenclature of X-ray Lines
Two nomenclatures are used to designate the X-ray lines. The older is based on the fixed intensity ratios of the individual lines and was introduced by Siegbahn (1923). It also shows the development of the performance of X-ray spectrometers, in particular their energy resolution. For the first instruments, only the distinction between K-, L-, and M-lines was possible. With the improvement of the resolution, a splitting of these lines was then discovered, i.e. α-, β-, and γ-lines could be distinguished. Later, further splittings were detected, which are denoted by indices.
There is also the nomenclature introduced by the IUPAC, which is based on an exact designation of the respective electron levels from which the X-ray lines are generated. A comparison of the designations for the most important X-ray lines can be found in Table 2.2.
2.2.4 Interaction of X-rays with Matter
X-radiation interacts with matter – it will be scattered and absorbed. This process is described by Lambert–Beer's law.
(2.5) 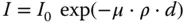
with
| I |
intensity after absorption in a layer |
| D |
thickness of the layer |
| P |
density of the layer |
| M |
mass attenuation coefficient of the layer material |
| I 0 |
primary intensity |
The mass attenuation coefficient μ has several contributions. At low energies, the absorption described by the photoionization coefficient τ is dominant; the influence of the scattering characterized by the scattering coefficient σ increases with energy, and in the case of energies >1.2 MeV the electron pair production described by κ gains importance. However, this is outside the range of energy that is of interest for X-radiation.
(2.6) 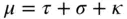
Читать дальше