Composition of the Dentin Matrix
The organic matrix of dentin contains collagen, non-collagenous proteins (proteoglycans, phosphophoryns, and glycoproteins), phospholipids, and growth factors.
Collagen
Type I collagen is the major protein of dentin matrix. Lesser amounts of types III, V, and VI collagen are also found in dentin matrix.
Electron microscopic autoradiographic studies with tritiated proline and immunocytochemical studies have shown that the procollagen of dentin matrix is secreted mainly from the predentinal segment of the odontoblastic process ( Figs 2-4to 2-6). 35 , 49Tritiated proline–labeled granules accumulate in the distal part of the cell body and are discharged by a process of merocrine exocytosis. A smaller number of labeled secretory granules are present in more distal parts of the process beyond the predentin. Presumably they are secreted at sites distal to the mineralization front.
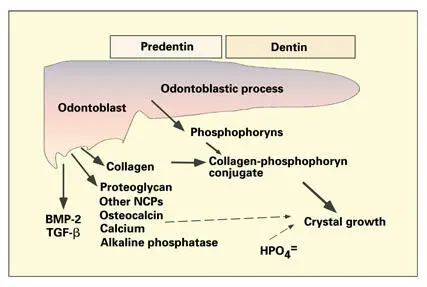
Fig 2-4Interaction of odontoblast secretory products in predentin, dentin, and the mineralization front. Phosphate ions in phosphophoryns sequester calcium and initiate the growth of hydroxyapatite crystals. The linkage of phosphophoryns and collagen leads to deposition of mineral along the collagen fibrils. A portion of the proteoglycans are degraded and removed from the predentin before mineralization of the collagenous matrix. Growth factors (bone morphogenetic protein 2 [BMP-2] and transforming growth factor β [TGF-β]) are retained in the matrix. (NCPs) Noncollagenous proteins. (Adapted from Veis. 226)
Following exocytosis of procollagen into the extracellular space, neutral proteinases remove the terminal amino and carboxy propeptides of the procollagen molecules, permitting collagen molecules to assemble into 64-nm banded fibrils of the predentin and dentin matrix (see chapter 6). Predentin matrix remains unmineralized for several days following its secretion. Typically, a layer of unmineralized predentin, approximately 10 to 20 μm thick, separates mineralized dentin from the cell body of the odontoblast (see Figs 2-2and 2-3). A widened predentin layer is usually a sign of abnormal mineral metabolism and/or matrix mineralization.
Fibronectin is also found in association with collagen fibrils in the predentin. Tissue inhibitor of matrix metalloproteinase 1, another secretory product of odontoblasts, is found in high concentration in predentin. 59
Figs 2-5a to 2-5dLight microscopic autoradiographs of the utilization of tritiated proline (reflecting collagen synthesis) by odontoblasts at various time periods after intravenous injection. (Original magnification × 500.)
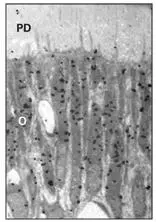
Fig 2-5aAt 10 minutes, the label is at the cell periphery. (PD) Predentin; (O) odontoblast.
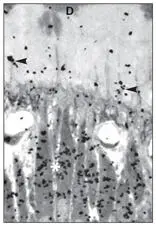
Fig 2-5bAt 20 minutes, the Golgi complex is heavily labeled. (arrowheads) Odontoblastic process; (*) approximate location of Golgi; (D) dentin.
Fig 2-5cAt 30 minutes, the grains are mostly over the odontoblastic process (arrowheads) .
Fig 2-5dAt 2 hours, most of the radioactivity is now in the predentin.
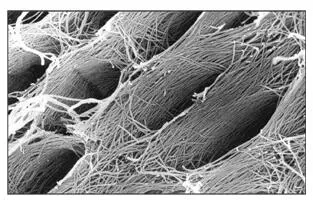
Fig 2-6aScanning electron microscopic view of the surface of predentin after the pulp and odontoblasts have been stripped away. The oval depressions represent the spaces (dentinal tubules) previously occupied by the odontoblastic processes. Note the uniform diameter (about 3 μm) of the collagen fibrils and their orientation around and perpendicular to the long axis of the dentinal tubule. (Adapted from Tabata et al 43with permission from John Wiley & Sons. Original magnification × 18,000.)
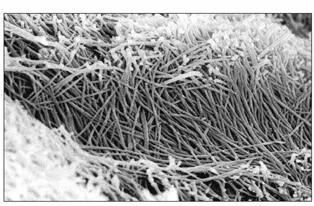
Fig 2-6bHigher magnification of the wall of the tubule in the predentin. No lamina limitans is present in predentin. Note the compact and woven arrangement of the collagen fibrils. These fibrils constitute the collagenous component of the intertubular dentin. (Adapted from Tabata et al 43with permission from John Wiley & Sons. Original magnification × 31,000.)
Noncollagenous proteins
Odontoblasts secrete noncollagenous proteins consisting of proteoglycans, phosphophoryns, and glycoproteins (see Fig 2-4). Electron microscopic autoradiographic localization of sulfate 35 and tritiated fucose have shown that the proteoglycan and glycosaminoglycan components of the matrix are concentrated at the mineralization front. 29 , 38 , 48 , 60 , 61
Biochemical and immunohistochemical studies indicate that there are specific differences in proteoglycan composition between predentin and dentin. 62 , 63Because proteoglycans interact with collagen during fibril formation, a function of predentin proteoglycans might be to regulate the size and orientation of dentin collagen fibers. It has also been suggested that predentin proteoglycans might control the timing and site of mineralization, either by sequestering calcium or by shielding potential mineral nucleation sites in the matrix. Evidence that some proteoglycans are degraded near the mineralization front by proteoglycanases and metalloproteinases supports the idea that some proteoglycans may indeed inhibit mineralization of the dentin matrix. 64 , 65
Decorin, a chondroitin–dermatan sulfate proteoglycan with binding affinity for type I collagen, is found in dental pulp, odontoblasts, at the mineralization front, and along the mineralized walls of the dentinal tubules. 66In contrast, decorin is conspicuously absent from predentin.
Porcine predentin matrix contains active neutral metalloproteinases (56- and 61-kDa gelatinases and 25-kDa proteoglycanase) capable of degrading proteoglycans at the mineralization front. 64 , 65The activity of these enzymes is calcium dependent. A mechanism must exist to regulate the availability of calcium for enzyme activation and matrix mineralization at the mineralization front. Endocytosis of proteoglycan degradation products, and membrane retrieval by coated vesicles, occurs in the proximal part of the odontoblastic process.
Not all of the matrix proteoglycans are degraded prior to mineralization. Chondroitin-6-sulfate, chondroitin-4-sulfate, and hyaluronate, associated with core proteins, have been extracted from demineralized dentin. Five dentin proteoglycans, ranging in size from 100 to 400 kDa and rich in chondroitin-4-sulfate, have been partially characterized by Steinfort et al. 63
Three noncollagenous proteins, dentin phosphophoryn (DPP), dentin matrix protein 1 (DMP1), and dentin sialoprotein (DSP), all contained in dentin matrix, appear to be specifically associated with biomineralization. The role of noncollagenous proteins in dentin formation has been the subject of recent reviews. 67 , 68
Читать дальше