I would like to thank all authors, companies, government agencies, international organizations and others who have contributed data and information to this book. The author has made every effort to acknowledge the contributors in the references section of each chapter and throughout the narrative.
Last but not least I would like to offer my sincere apologies for any errors the readers may find in this book. The errors are the responsibility of the author. A note to the publisher would be most appreciated so that corrections could be made in the future editions.
I would like to thank Scrivener Publishing for the contracting and production of the book and John Wiley & Sons for its distribution and marketing.
Sina Ebnesajjad, PhD www.FluoroConsultants.comChadds Ford, Pennsylvania January 2, 2021
Dictionaries define fluorocarbons as any of various chemically inert compounds containing mainly carbon and fluorine. They are used to produce refrigerants, fire extinguishing and foaming agents, aerosol propellants, polymers, nonstick coatings and lubricants. In the industry parlance the term fluorochemicals is often used instead of fluorocarbon. Rather fluorocarbons usually refer to small molecule fluorinated gases and liquids. The magic in these compounds of all sizes is in the carbon fluorine bond (C-F). It has a dissociation energy of 536 kJ/mol ranking as the single strongest carbon bond [1–4]. The replacement of C-H bond in hydrocarbons with C-F is the single most important factor giving rise to the unique properties of fluorocarbons.
In this book the terms fluorochemicals and fluorocarbons refer to all aliphatic organic compounds containing fluorine and carbon, i.e. fluorinated chemicals. Aliphatic is defined as straight chain, branched chain, or cyclic, saturated, as in the paraffin; or unsaturated, as in the olefins and alkynes. Cyclic fluorocarbons are not discussed in this book because their limited commercial applications.
More specifically the term fluorocarbon refers to small molecule of fluorinated alkane and olefin compounds. Fluorinated polymers and fluoropolymers are used to refer to, mostly, olefinic macromolecules of fluorinated thermoplastics and elastomers. Partially fluorinated refers to a material that contains residual C-H or other bonds. Perfluorinated refers to the absence of hydrogen in a chemical due to complete fluorine substitution. Other atoms such as oxygen, sulfur and nitrogen may be present in the structure of the perfluorinated chemicals, often in small quantities.
Commercial is the guiding beacon for the selection of the contents of the present book. The focus is on fluorochemicals produced and consumed in commercial applications. An important aspect of the commercial consumption of fluorocarbons is their impact on the ozone layer as a result of emissions of those chemicals. The issue came to surface when chlorofluorocarbons were discovered to deplete atmospheric ozone layer. That discovery has resulted in decades of development of replacement compounds and elimination of all ozone depleting fluorocarbons.
The three major groups of commercial products consumed in large quantities are listed in Table 1.1. Of the major fluorinated organic products, only fluorocarbons have small molecules and interact with the ozone layer. Some of the precursors of fluoropolymers and fluoroelastomers impact the ozone layer thus must be contained while being converted to polymers.
Table 1.1Examples of commercial fluorochemicals.
| Product |
Size of molecule |
Example of compound |
Example of application |
| Fluorocarbons |
Small |
HFC-134a (1,1,1,2-tetrafluoroethane) |
Refrigerant, Propellant for aerosols, Blowing agent for foams |
| Thermoplastic Fluoropolymers (TPF) and Fluoroelastomers (FE) |
Large macromolecules |
Polyvinylidene fluoride (TPF), copolymer of vinylidene fluoride/tetrafluoroethylene |
Tubes, pump bodies (TPF), seals and gaskets (FE) |
| Fluorinated Coating - Dispersion (D), powder (P) |
Large macromolecules |
Polytetrafluoroethylene coatings,Tetrafluoroethylene/perfluoroalkylvinylether copolymer powder |
Interior surface of oil pipelines (D),Corrosion resistant (P) |
The terms ozone depletion refer to the destruction of atmospheric ozone by the decomposition products of certain gases. Ozone by its action filters out low wavelength ultraviolet light waves known as “UV B” which are hazardous to humans, and some plants and wildlife. Destruction of the atmospheric ozone reduces its filtration action thus allowing an increase in the UV B light waves that reach the earth surface. The First generation of fluorocarbon gases were discovered to be strong causes of ozone depletion. A great deal of global regulatory changes, research, publication, development and replacement have taken place since the 1980s. These issues are discussed in detail in the rest of this book.
Most public and technical discussions about ozone layer and depletion are not quantitative. Nevertheless, atmospheric ozone has been measured to allow factual analysis of changes in the ozone layer. The Dobson Unit (DU) is the unit of measure for total ozone ranging from 100 to 500. If one were to take all the ozone in a column of air stretching from the surface of the earth to space (Figure 1.1) and bring all that ozone to 0°C and pressure of one atmosphere, the column would be about 0.3 centimeters thick. Thus, the total ozone would be 0.3 atm-cm. To make the units easier to work with, the “Dobson Unit” is defined to be 0.001 atm-cm, 0.3 atm-cm would be 300 DU [5].
Incidentally, Dobson Unit is named after Gordon Miller Bourne Dobson (1889-1976) who was a British physicist and meteorologist. Professor Dobson was an ingenious experimentalist who devoted much of his life to the observation and study of atmospheric ozone. He made measurements at a number of locations in Europe to study the relation between ozone distribution and synoptic meteorological variables. The results of Dobson’s studies turned out to be of great importance. They lead to an understanding of the structure and circulation of the stratosphere including the ozone layer [7].
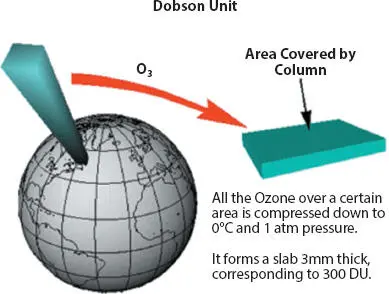
Figure 1.1Depiction of Dobson Unit for ozone quantitative measurement [6].
1.2 Production and Consumption Statistics of Fluorocarbons
Statistics on the production and consumption of fluorocarbons illustrate the continued growth of those products. A major study published by the US Government in February 2020 sheds light on the global development, trade and consumption of fluorocarbon gases and their alternatives. This study is the result of collaboration of Department of Energy’s National Renewable Energy Laboratory and Oak Ridge National Laboratory [8] Some of the highlights of this study are described in the next Section (1.2.1).
1.2.1 Refrigerants: Market Trends and Supply Chain Assessment
This section has been adopted with some modifications from a Department of energy study published in May 2020 [8]. The conclusions of the reported study are listed in this section. The term refrigerants has a broad meaning in this report because most of the refrigeration gases are also used in non-refrigeration applications.
Читать дальше













