At the leading side of the tooth the negative strain stimulates the secretion of RANKL, but decreases the secretion of OPG, and thus the differentiation and functioning of osteoclasts are stimulated. On the other hand, in the areas with positive strain, the trailing side of the tooth, RANKL as well as OPG are upregulated, but OPG is more upregulated than RANKL, and thus osteoclast differentiation is prevented (Hadjidakis and Androulakis, 2006; Yamaguchi, 2009; Vansant et al ., 2018).
For the functioning of osteoclasts, they should be attached to mineralized bone matrix through αVβ3 integrin. This is only possible when the osteoblasts, as well as the osteoid, the nonmineralized bone matrix covering the surface of the alveolar bone, are removed (Duong et al ., 2000; Takahashi et al ., 2007; Eriksen, 2010). The ECM of the osteoid is degraded through the action of MMPs, more specifically the collagenases MMP1, MMP8, MMP13, and MMP14 (Tokuhara et al ., 2019). These enzymes are synthetized and secreted as pro‐enzymes by a variety of cell types, including lymphocytes and granulocytes, but in particular by activated macrophages. They are activated by proteolytic cleavage and regulated by a family of inhibitors called the tissue inhibitors of matrix metalloproteinases (TIMPs). The MMP activity is thus dependent on the balance between production and activation of MMPs and the local levels of TIMPs (Snoek‐van Beurden and Von den Hoff, 2005; Verstappen and Von den Hoff, 2006; Tokuhara et al ., 2019). The osteoblasts disappear by apoptosis (programmed cell death), induced by binding of TNF‐α (that is secreted by activated macrophages, fibroblasts, and osteoblasts, in an autocrine way) to its receptors TNFR1 and TNFR2 on osteoblasts and the subsequent activation of the caspase pathway (Hill et al ., 1997; Jilka et al ., 1998; Hock et al ., 2001).
The combined osteoblast apoptosis and ECM degradation leads to areas of exposure of mineralized bone matrix, which can serve as landing sites for osteoclasts. The osteoclasts move to the landing sites by chemotaxis, and attach to the bone by αVβ3 integrins, connecting the osteoclast to RGD peptides in the bone matrix (Takahashi et al ., 2007; Lerner et al ., 2019). Upon adhesion to bone, osteoclasts polarize and reorganize their cytoskeleton to generate a ring‐like F‐actin‐rich structure, the sealing zone, that isolates the Howship’s lacuna from the surroundings. Inside the sealing zone, the ruffled border is formed. The isolated area becomes acidic through an H +‐ATPase‐mediated proton pump. This favors the dissolution of bone minerals. In addition, the lysosomal enzyme cathepsin K, a cysteine proteinase with a pH optimum of 4.5, and matrix metalloproteinases, especially MMP‐9 (pH optimum = 7.4) are secreted into Howship’s lacunae to degrade the organic bone matrix (Teitelbaum, 2000) ( Figure 3.14).
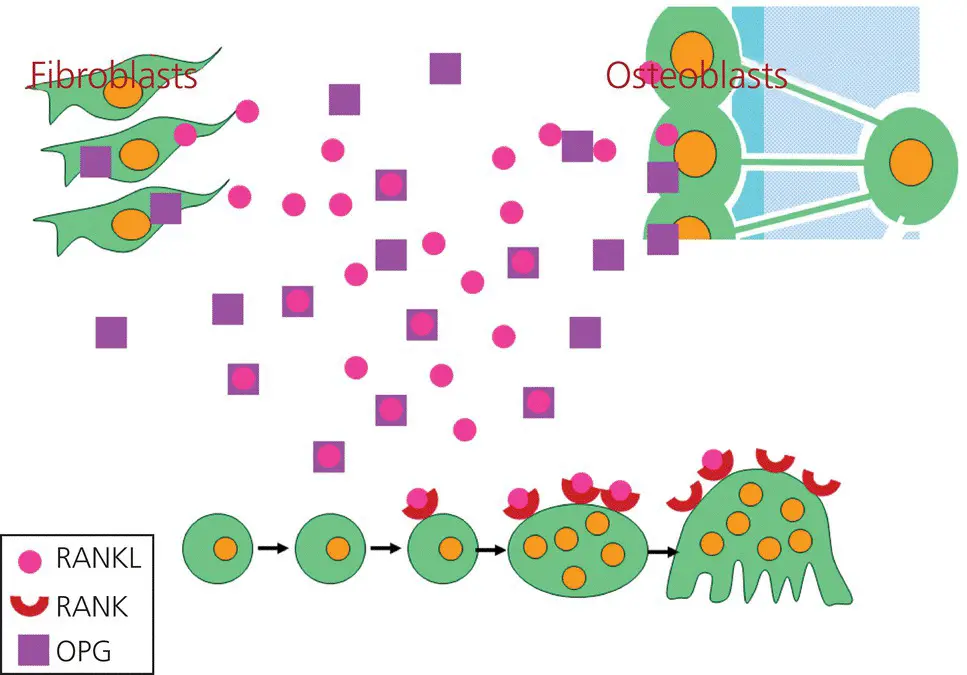
Figure 3.13 The RANK/RANKL/OPG system. The RANKL that is secreted by fibroblasts and osteoblasts binds to RANK, expressed on the osteoclast precursors. The latter subsequently become mononuclear osteoclasts. After fusion, these cells become multinuclear osteoclasts. However, fibroblasts and osteoblasts also can secrete osteoprotegerin (OPG), a soluble factor that also binds to RANKL, thereby hampering the differentiation of osteoclasts.
(Source: Jaap Maltha.)
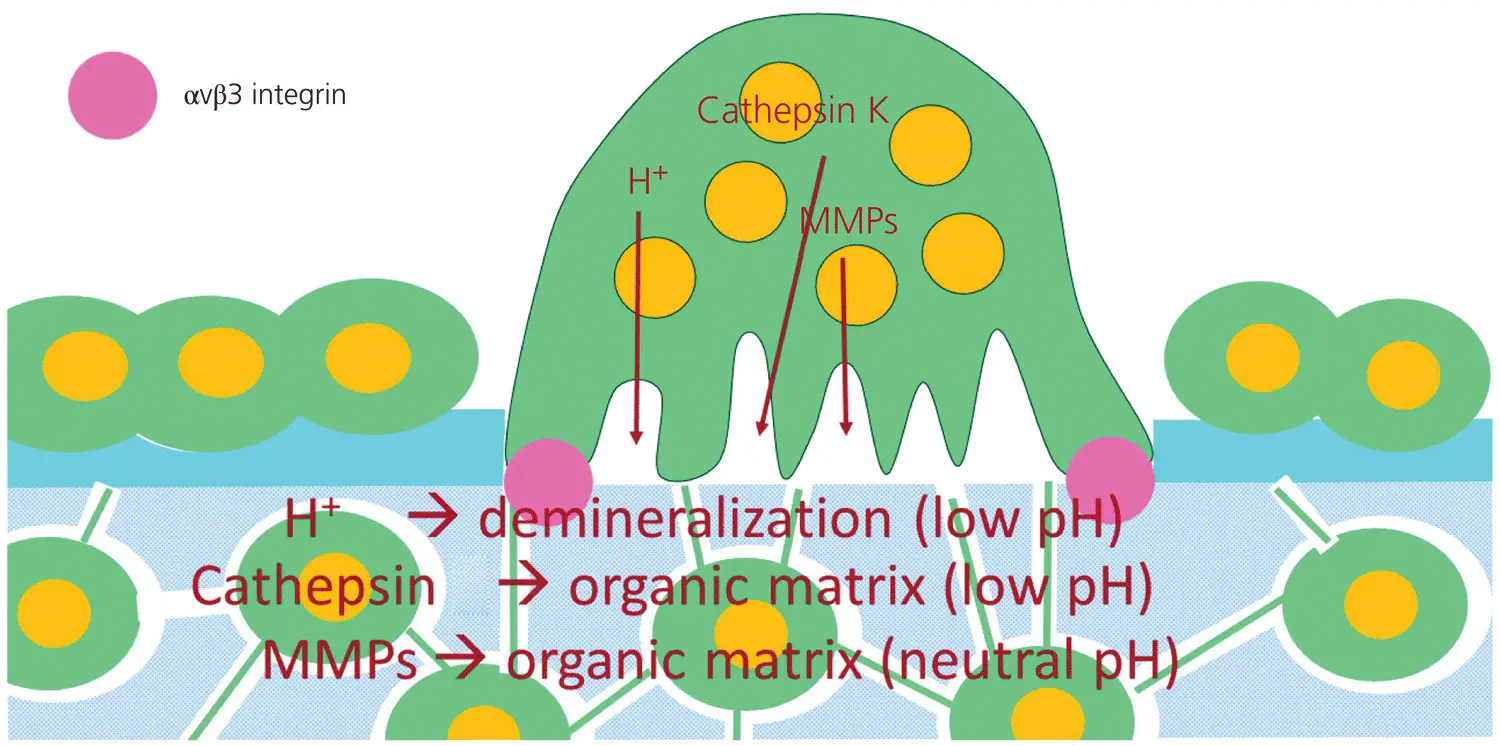
Figure 3.14 An active osteoclast.
(Source: Jaap Maltha.)
At the trailing side of the moving tooth, the PDL is widened, accompanied with a positive strain in the ECM and an acute inflammatory reaction ( Figure 3.15). This results in an increase in IL‐1β, IL‐10, PGE2, and TGF‐β expression (Tsuge et al ., 2016; Li et al ., 2018), and subsequently in an increase in OPG and a decrease in RANKL secretion by the osteoblasts and periodontal fibroblast (Li et al ., 2018).
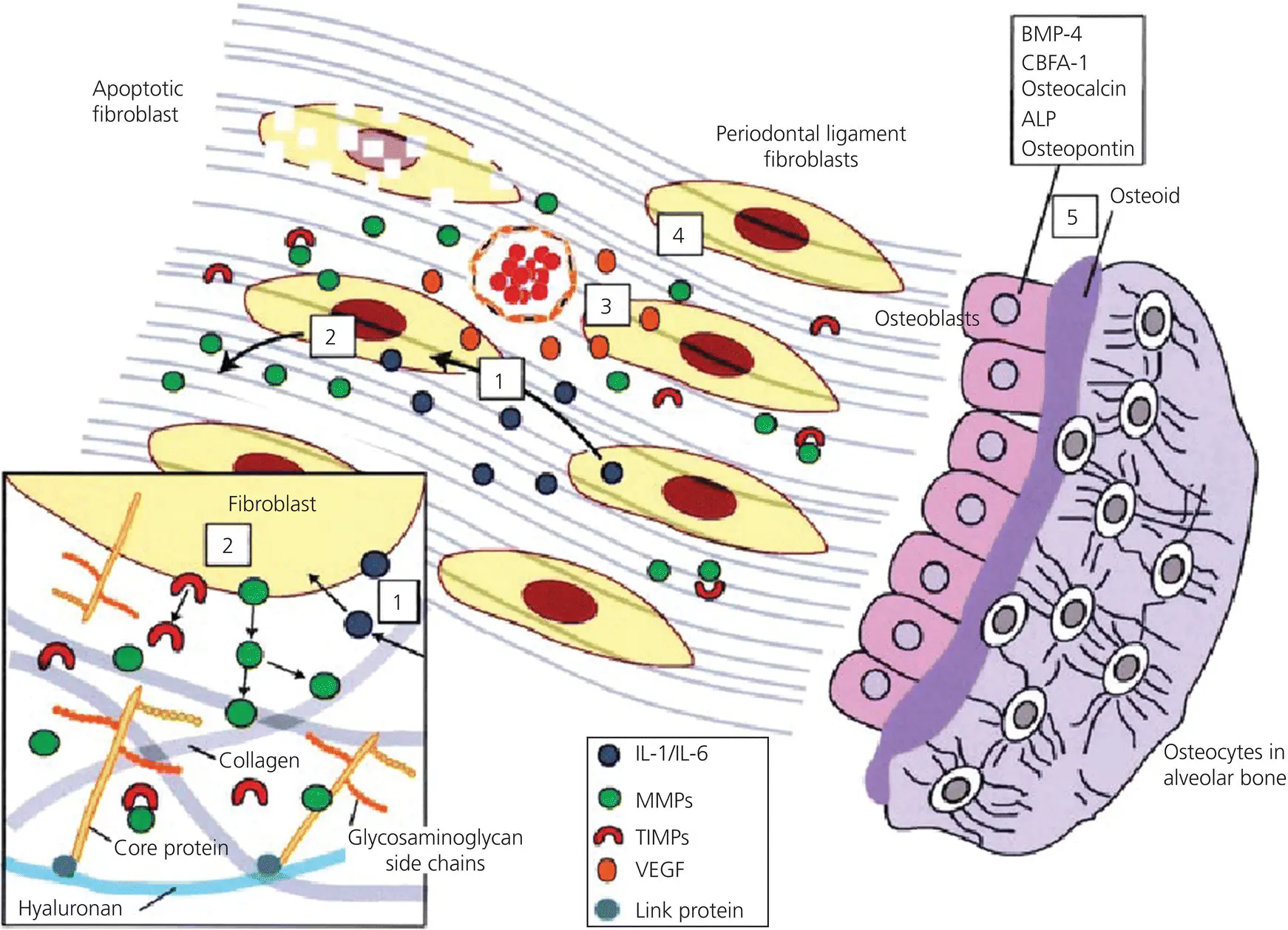
Figure 3.15 Summary of the remodeling processes at the trailing side. Fibroblasts under tensile strain secrete IL‐1 and IL‐6 (1), which in turn stimulate MMPs and inhibit TIMPs (2). Fibroblasts also secrete VEGF that stimulates angiogenesis (3). These actions result together in anabolic activities of fibroblasts (4) and osteoblasts (5).
(Source Meikle, 2006. Reproduced with permission of Oxford University Press.)
Furthermore, the number of fibroblasts increases, and the secretion of collagen type I and collagen type III, as well as the formation of new Sharpey’s fibers, is stimulated. Simultaneously with the deposition of the Sharpey’s fibers, osteoblasts deposit new bone matrix on the adjacent alveolar bone socket wall, anchoring the Sharpey’s fibers in the bone matrix (Garant and Cho, 1979; Militi et al ., 2019). On the other hand, the expression of MMPs is downregulated and the expression of TIMPs is upregulated, and thus ECM breakdown is inhibited.
Finally, FGF‐2 and VEGF, growth factors involved in the development of vascular elements, are upregulated (Chen et al ., 2014; Salomão et al ., 2014; Li et al ., 2018; Militi et al ., 2019).
The cumulative result is that at the trailing side osteoclast differentiation is prevented, the formation of new ECM and bone deposition is stimulated, and adaptation of the vascular system to the new situation is induced.
Cell biological processes during relapse and retention
It is generally accepted that if the orthodontic appliance is removed, the teeth tend to revert back in the direction of their original position by a process called relapse. This starts almost immediately after removal of the appliance and the rate of the relapse decreases over time. This process can be described as a logarithmic decay curve, with a half‐life time (T½) of approximately 1–11 days and stabilization after approximately 10 weeks (Maltha and Von den Hoff, 2017).
The classic theory is that relapse is caused by the relaxation of stretched fibers in the PDL and/or the supra‐alveolar region (Littlewood et al ., 2017). However, recent studies have shown that the turnover rate of the collagen fibers in the normal PDL and the supra‐alveolar region is very high. Their T½ varies between 3 and 10 days, which means for example that after about 3 months only 0.2% of the original fibers still remain (Henneman et al ., 2012). Furthermore, within a few days after the start of the active treatment, the structure of the PDL at the leading side is completely remodeled, while at the trailing side, part of the original fibers are embedded in the alveolar bone, and newly synthetized collagen fibers bridge the gap to the moving tooth (Von Böhl et al ., 2004a; Nakamura et al ., 2008; Tsuge et al ., 2016).
This indicates that the classic theory should be rejected. It is more likely that the relapse is initiated by the changes in the mechanical conditions in the PDL due to the abolition of the external force. This leads to changes in the stress and strain distribution in the PDL, which in turn will induce changes in the synthesis and release of molecules that modulate the differentiation, proliferation, and activation of cells in the PDL, the alveolar bone, and the cementum.
Читать дальше