In the United States, new chemicals are regulated by the US EPA Toxic Chemicals Control Act (TSCA), amended by the LCSA in June 2016. When a new chemical substance, as defined by the TSCA regulations is to be put into commerce there are specific data and information requirements for submittal and approval to the EPA. The EPA must in essence approve the commercial production of the chemical based on the data provided and the known uses, exposures, and releases for the product.
For the research phase of new chemicals, there are actually a number of regulatory exceptions in terms of hazard assessment, hazard communication, and provision of information in the more limited supply chain of research and development.

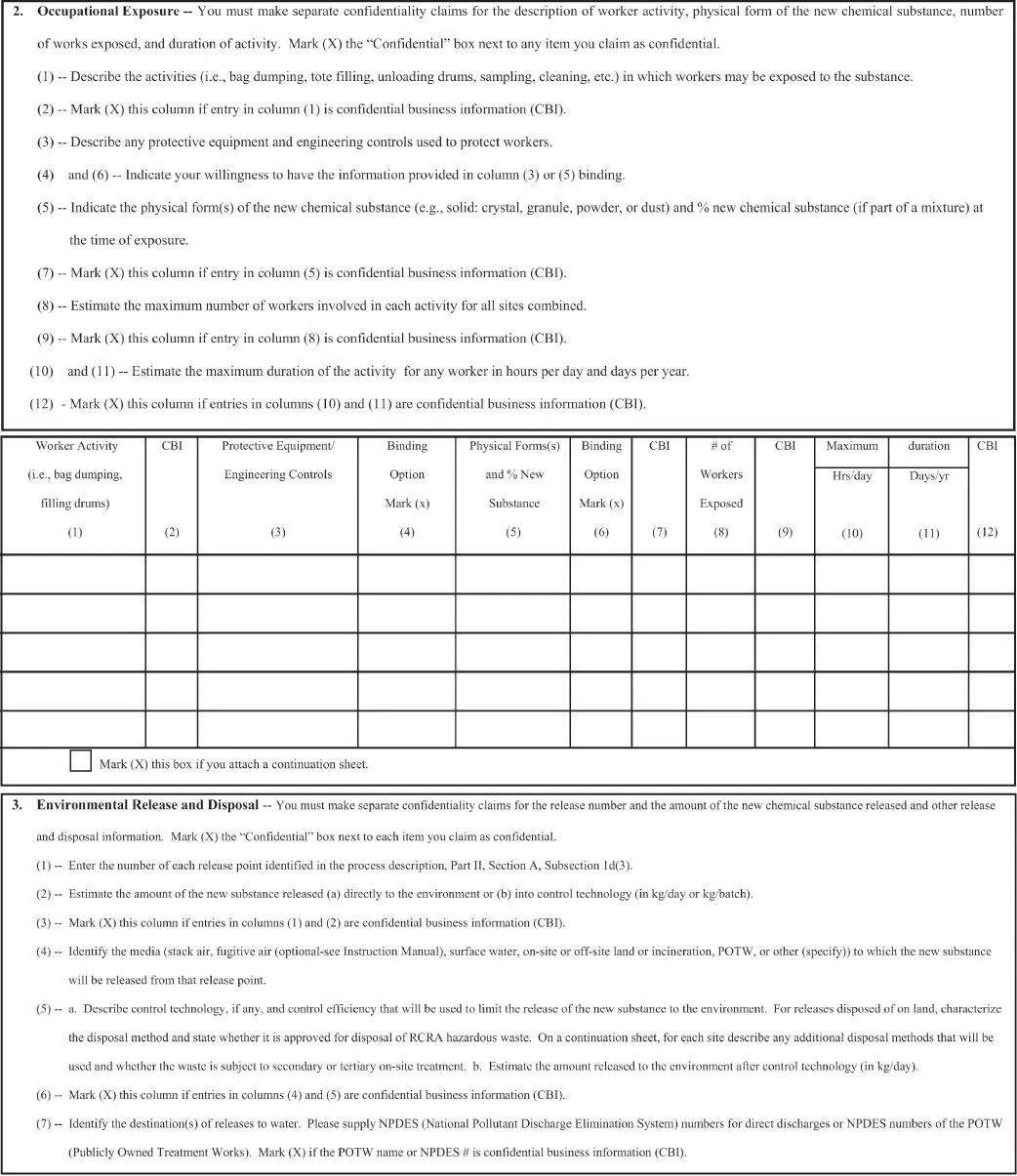

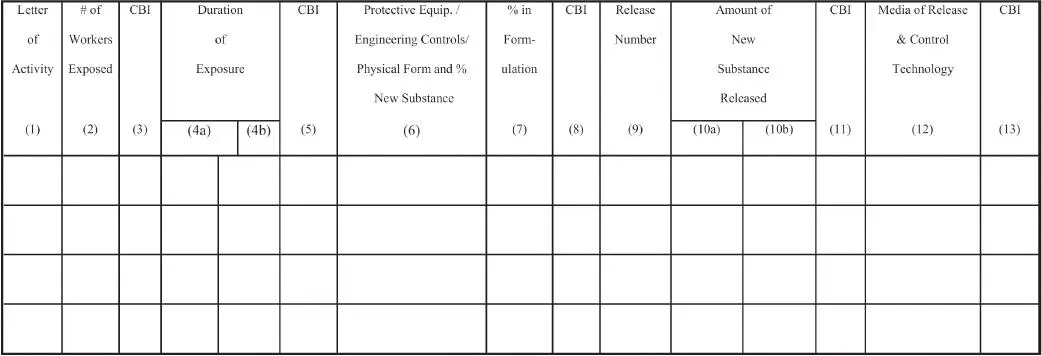
When a manufacturer moves to the point of gaining approval for commercial activity by submitting a Premanufacture Notification (PMN) for a new chemical, PS is evidenced in the PMN form. In Part II of the form, Human Exposure and Environmental Release, submitters have the opportunity to describe use and exposure conditions in their workplace as well as in the sites not controlled by them, specifically the customer or chemical user workplace. The more accurate and precise the information, the better the results of the EPA review can be. Doing a good job in understanding exposure and use can lower the potential for testing requirements or risk management controls such as a significant new use rule (SNUR). The applicable portions of the form are shown below. The PMN form requirements are found in EPA regulations, 40 CFR Part 723 (5).
In 2007, there were 10 countries around the world that had a similar form of chemical control regulation. In 2018, there were over 15 countries and regions. In addition to the United States, they are Australia, Canada, China, European Union, Japan, Korea, New Zealand, Philippines, and Switzerland.
3.10 US EPA Chemical Data Reporting Rule
The Chemical Data Reporting (CDR) rule, under the TSCA, requires manufacturers (including importers) to provide EPA with information on the production and use of chemicals in commerce in large quantities (6).
Under the CDR rule, EPA collects basic exposure‐related information on the types, quantities and uses of chemical substances produced domestically and imported into the United States. It constitutes the most comprehensive source of basic screening level and exposure‐related information on chemicals available to EPA, and is used by the Agency to protect the public from potential chemical risks.
The information is collected every four years from manufacturers (including importers) of certain chemicals in commerce generally when production volumes for the chemical are 25 000 pounds or greater for a specific reporting year. Collecting the information every four years assures that EPA and (for non‐confidential data) the public have access to up‐to‐date information on chemicals that are produced in large quantities.
The CDR rule is required by section 8(a) of the TSCA, and was formerly known as the Inventory Update Rule (IUR).
Read the TSCA CDR requirements in the Code of Federal Regulations (40 CFR Part 711).
3.10.1 Exposure‐Related Information Required
Examples of EPA data requirements involving exposure information are: (i) the number of workers “reasonably likely to be exposed” to the chemical at the manufacturing site; (ii) the physical form(s) in which the chemical substance is sent off‐site; (iii) the percentage of total reported production volume associated with each physical form; and (iv) the maximum concentration of the chemical substance at the time it leaves the submitter's manufacturing site or, if the chemical substance is site‐limited, the maximum concentration at the time it is reacted on‐site to produce a different chemical substance; (v) whether the use is consumer or commercial; (vi) if the chemical is used in products intended for children. “Reasonably likely to be exposed” means an exposure to a chemical substance which, under foreseeable conditions of manufacture, processing, distribution in commerce, or use of the chemical, is more likely to occur than to not occur. Covered exposures include exposures through any route of entry, but exclude “accidental” or “theoretical” exposures.
The CDR requirements increase the need to have certain exposure information pertinent to chemical substances in the workplace and in commerce, or during use. Producers will need more detailed information about workplace exposures and producers making more than 300 000 pounds per year per chemical substance will need specific processing and use information as required under the rule. EPA states that its three primary reasons for seeking such information are to tailor a chemical substance's reporting requirements more closely to match EPA's information needs; to obtain new and updated information relating to potential exposures to a subset of chemical substances listed on the TSCA Inventory; and to improve the utility of the information reported. The agency has stated that receipt of this exposure information will “enable EPA to more selectively conduct initial risk screening on a subset of the chemical substances with its purview.” These data will, along with other EPA initiatives to collect hazard data, including the High Production Volume (HPV) Challenge Program and Voluntary Children's Chemical Evaluation Program (VCCEP), allow EPA to prioritize its TSCA Section 4rulemaking and enforceable consent agreement initiatives. This verbiage emphasizes the increasing need for exposure information and the involvement of IH in this regulatory process.
3.11 European Chemical Control Regulations: Registration, Evaluation, and Authorization of Chemicals (REACHs)
In June 2007, a new set of regulatory requirements for the REACH substances came into force (Regulation (EC) No. 1907/2006) (7). This is the most comprehensive set of chemical regulations to be enacted in the EU and the impact of these regulations will be global in nature. In the future, based on a specified set of deadlines all substances produced, sold, or imported in the EU will have to be registered. The regulations remove the distinction between new and existing substances and require the same level of comprehensive data to be submitted for all substances with the amount of information varying based on volume and inherent hazard.
For all substances produced above 10 tons annually, a chemical safety assessment will have to be submitted as part of the required information. For substances determined to be dangerous, a chemical safety report (CSR) must be developed and that CSR must describe exposure scenarios. Exposure scenarios are sets of conditions that describe how substances are manufactured and used during manufacture and ultimate use by the downstream users. The CSR must also have information on how the manufacturer or importer controls or recommends to control exposures to workers and the environment. The exposure scenarios must also include recommended risk management measures for all “identified uses.” These exposure scenarios and risk management measures will then need to be annexed in some way to MSDSs.
Читать дальше
















