Ecological surveys carried out at the Bordeaux Faculty of Enology from 1992 to 1999 (Naumov et al ., 2000a) demonstrated the presence of S. uvarum yeasts on grapes and in spontaneously fermenting white musts from the Loire Valley, Jurançon, and Sauternes. The frequency of the presence of this species alongside S. cerevisiae varies from 4 to 100%. On one estate in Alsace, strains of S. uvarum were identified on grapes, in the press, and in tanks, where they represented up to 90% of the yeasts involved throughout fermentation in three consecutive years (Demuyter et al ., 2004). More recently, other authors (Naumov et al ., 2002; Zhang et al ., 2015) have shown that S. uvarum , identified on grapes and in fermenting must, is involved in making Tokaji and New Zealand wines.
The adaptation of S. uvarum to relatively low temperatures (6–10°C) certainly explains its presence in certain ecological niches: northerly vineyards, late harvests, and spontaneous “cold” fermentation of white wines. In contrast, this strain is sensitive to high temperatures and has not been found in spontaneous fermentations of red Bordeaux wines.
Between two harvests, winery walls, floors, equipment, and sometimes even the winery building itself are colonized mostly by the various non‐fermentation species previously cited. Winemakers believe, however, that spontaneous fermentations are more difficult to initiate in new tanks than in tanks that have already been used. This empirical observation leads to the supposition that S. cerevisiae can also survive in the winery between two harvests. Moreover, this species was found in non‐negligible proportions in the wooden fermentors of some of the best vineyards in Bordeaux during the harvest, just before they were filled.
Recent studies relying on global approaches to sequencing of DNA extracted from a biological sample demonstrate the presence of the main yeast species in fermenting must ( H. uvarum , S. bacillaris , and S. cerevisiae ) in the various zones of winemaking cellars (pressing, fermentation, and storage) before, during, and after the harvest period (Bokulich et al ., 2013).
In the first hours of spontaneous fermentations, the first tanks filled have a very similar microflora to that of the grapes, with a large proportion of H. uvarum , S. bacillaris (formerly known as Candida zemplinina ), and M. pulcherrima species. After about 20 hours, S. cerevisiae develops and coexists with the grape yeasts (Zott et al ., 2008). Non‐ Saccharomyces yeasts quickly disappear at the start of spontaneous fermentation ( Figure 1.35). The microflora of tanks inoculated with yeast is very similar to that of tanks undergoing spontaneous fermentation ( Figure 1.36). The major difference resides in the nature of S. cerevisiae strains that conduct fermentation: there is a dominant clone in the case of adding yeast. Meanwhile, in the case of spontaneous fermentation, several clones coexist ( Section 1.10.2). Thus, the practice of adding yeast does not eliminate populations of non‐ Saccharomyces yeasts at the start of alcoholic fermentation. In red winemaking in the Bordeaux region, as soon as must specific gravity drops below 1.070–1.060, the colony samples obtained by plating diluted must on a solid medium generally isolate S. cerevisiae (10 7–10 8cells/ml) exclusively. This species plays an essential role in the alcoholic fermentation process. Environmental conditions influence its selection. This selection pressure is exhibited by four main parameters: anaerobic conditions, must or grape sulfiting, sugar concentration, and the increasing presence of ethanol. The increase in temperature, especially in the case of red winemaking, also favors the development of S. cerevisiae to the detriment of non‐ Saccharomyces yeasts (Goddard, 2008). In winemaking, where no sulfur dioxide is used, such as white wines for the production of spirits, the dominant grape microflora can still be found. It is largely present at the beginning of alcoholic fermentation ( Figure 1.35). Even in this type of winemaking, the presence of apiculate yeasts is limited at the midpoint of alcoholic fermentation.
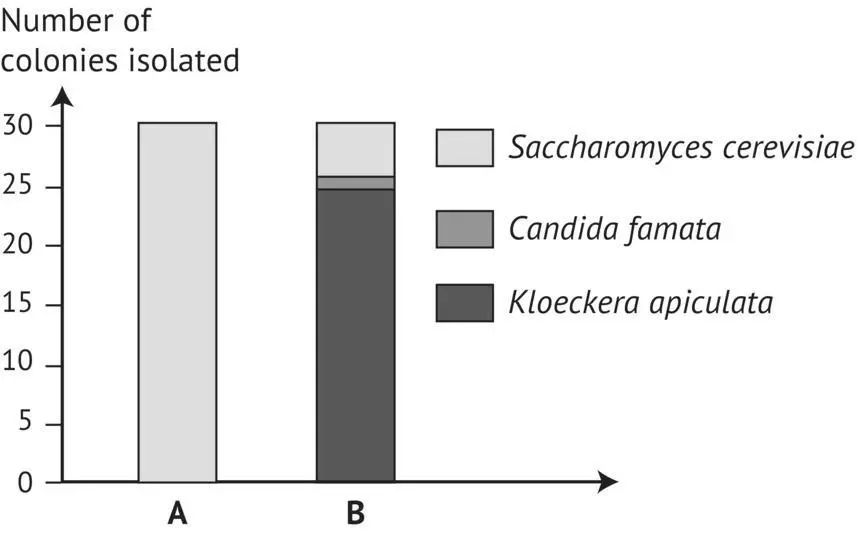
FIGURE 1.35 Comparison of yeast species present at the start of alcoholic fermentation ( d = 1.06). A, in a tank of sulfited red grapes in Bordeaux (Frezier, 1992); B, in a tank of unsulfited white must, for the production of Cognac (Versavaud, 1994).
During dry white winemaking, the separation of the pomace after pressing, combined with clarification by racking, greatly reduces yeast populations, at least in the first few days of the harvest. The yeast population of a severely racked must rarely exceeds 10 4–10 5cells/ml.
A few days into the harvest, the S. cerevisiae yeasts colonize the harvest equipment, grape transport machinery, and especially the grape receiving equipment, the crusher, stemmer, the wine press, and cellar atmosphere (Grangeteau et al ., 2015). For this reason, S. cerevisiae is already widely present at the time of filling the tanks (around 50% of yeasts isolated during the first homogenization pump‐over of a red grape tank). Fermentations are initiated more rapidly as harvest goes on. In fact, the last tanks filled often complete their fermentations before the first ones. Similarly, static racking in dry white winemaking becomes more and more difficult to achieve, even at low temperatures, from the second week of the harvest onward, especially in hot years. The entire facility inoculates the must with a sizeable fermentation yeast population. General weekly disinfection of the pumps, piping, wine presses, settling tanks, etc., is therefore strongly recommended.
During the final part of alcoholic fermentation (the yeast decline phase), the population of S. cerevisiae progressively decreases while still remaining greater than 10 6cells/ml. Under favorable winemaking conditions, characterized by a rapid and complete exhaustion of sugars, no other yeast species significantly appears at the end of fermentation. Under poor conditions, spoilage yeasts can contaminate the wine. One of the most frequent and most dangerous contaminations is due to the development of B. bruxellensis , which is responsible for serious off‐odors (Volume 2, Section 8.4.5).
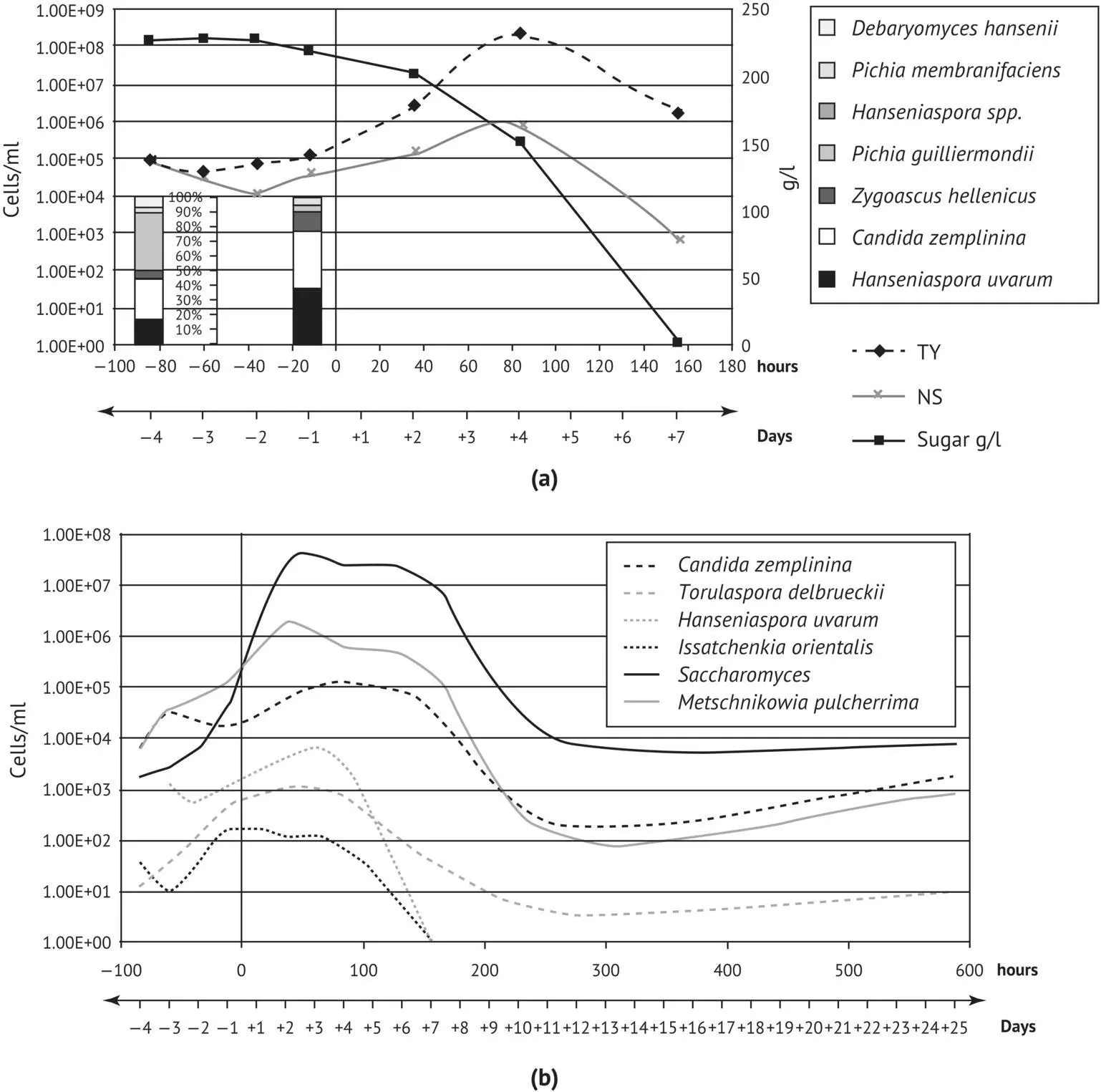
FIGURE 1.36 Dynamics of total yeasts and non‐ Saccharomyces yeasts during red winemaking monitored by (a) culture/RFLP‐ITS‐PCR and (b) specific quantitative PCR applied on a DNA pellet extracted directly from fresh must. Hour 0/day 0, time of inoculation with commercial yeast; − hours/days, cold soaking; + hours/days, alcoholic fermentation (Zott et al ., 2010).
In the weeks that follow the completion of alcoholic fermentation, the viable populations of S. cerevisiae drop rapidly, falling below a few hundred cells/ml. In many cases, other yeast species (spoilage yeasts) can develop in wines during bulk or bottle aging. Some yeasts have an oxidative metabolism of ethanol and form a veil on the surface of the wine, such as Pichia or Candida , or even certain strains of S. cerevisiae —sought after in the production of specialty wines. By topping up regularly, the development of these respiratory metabolism yeasts can be prevented. Some other yeasts, such as Brettanomyces or Dekkera , can develop under anaerobic conditions, consuming trace amounts of sugars that have been incompletely or not fermented by S. cerevisiae . Their population can attain 10 4–10 5cells/ml in a contaminated red wine in which alcoholic fermentation is otherwise completed normally. These contaminations can also occur in the bottle. Lastly, refermentation yeasts can develop significantly in sweet or botrytized sweet wines during aging or bottle storage. The principal species found are S. ludwigii , Z. bailii , and also some strains of S. cerevisiae that are particularly resistant to ethanol and sulfur dioxide.
Читать дальше














