Recent Advances in Polyphenol Research
Здесь есть возможность читать онлайн «Recent Advances in Polyphenol Research» — ознакомительный отрывок электронной книги совершенно бесплатно, а после прочтения отрывка купить полную версию. В некоторых случаях можно слушать аудио, скачать через торрент в формате fb2 и присутствует краткое содержание. Жанр: unrecognised, на английском языке. Описание произведения, (предисловие) а так же отзывы посетителей доступны на портале библиотеки ЛибКат.
- Название:Recent Advances in Polyphenol Research
- Автор:
- Жанр:
- Год:неизвестен
- ISBN:нет данных
- Рейтинг книги:5 / 5. Голосов: 1
-
Избранное:Добавить в избранное
- Отзывы:
-
Ваша оценка:
- 100
- 1
- 2
- 3
- 4
- 5
Recent Advances in Polyphenol Research: краткое содержание, описание и аннотация
Предлагаем к чтению аннотацию, описание, краткое содержание или предисловие (зависит от того, что написал сам автор книги «Recent Advances in Polyphenol Research»). Если вы не нашли необходимую информацию о книге — напишите в комментариях, мы постараемся отыскать её.
Recent Advances in Polyphenol Research
Recent Advances in Polyphenol Research — читать онлайн ознакомительный отрывок
Ниже представлен текст книги, разбитый по страницам. Система сохранения места последней прочитанной страницы, позволяет с удобством читать онлайн бесплатно книгу «Recent Advances in Polyphenol Research», без необходимости каждый раз заново искать на чём Вы остановились. Поставьте закладку, и сможете в любой момент перейти на страницу, на которой закончили чтение.
Интервал:
Закладка:
Table 1.2 Rate constants between AH+and CB(estimated error 10%). Reproduced from Mendoza et al. (2018), with permission.
Source: Mendoza et al. 2018
| k h/ s ‐1 | k ‐h/ M ‐1s ‐1 | k t/ s ‐1 | k ‐t/ s ‐1 | k i/ s ‐1 | k ‐i/ s ‐1 | |
|---|---|---|---|---|---|---|
| HBA1 | 0.01 | 377 | 0.09 | 0.086 | 2x10 ‐6 | 5x10 ‐7 |
| HBA2 | 0.12 | 145 | 0.08 | 0.23 | — | — |
| HBA3 | 0.35 | 43 | 0.12 | 0.33 | — | — |
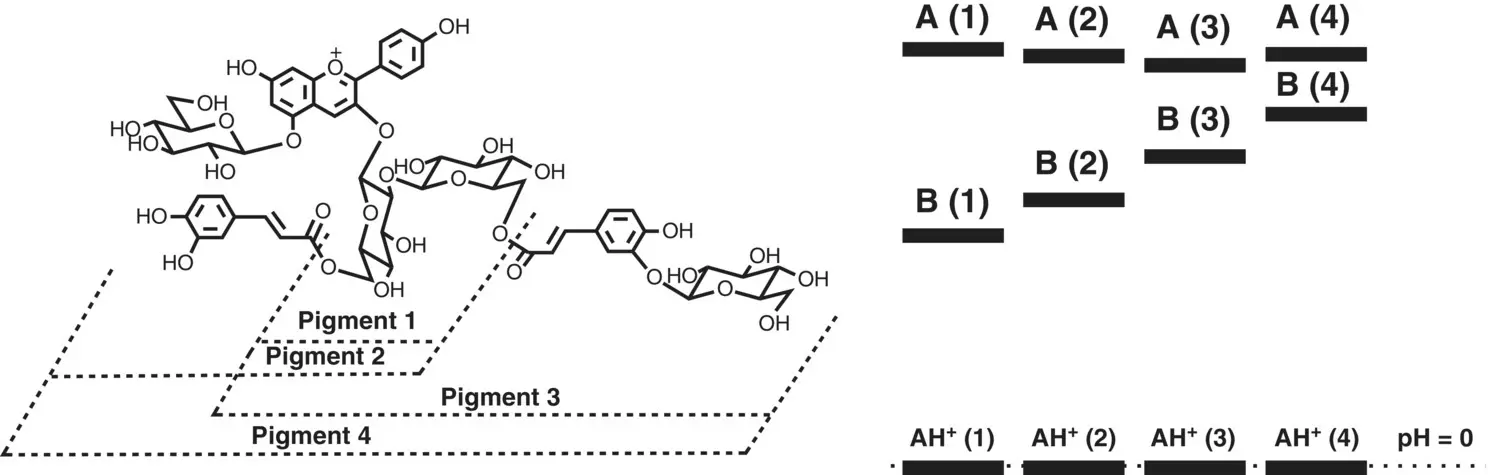
Scheme 1.9 (Left) The polyacylated anthocyanins (Dangles et al. 1993); (Right) representation of the relative energy levels at equilibrium using the hydration and acidity constants reported by the authors. The construction of this type of energy level diagram is shown in Scheme 1.2. It is worth noting that two acylated units are not enough to invert the energy levels between the hemiketal (B) and the quinoidal base (A).
Source: Dangles et al. 1993.
The protection effect of the acylated sugars for the hydration reaction was studied previously (Fernandes et al. 2015; Dangles et al. 1993), but according to our knowledge no other study of the physical chemistry of three acylated sugars has been reported. The most complete study previously published, regarding this type of molecules, was reported by Dangles et al. (1993) ( Scheme 1.9). In this work, the proton transfer as well as the hydration constants were determined, allowing the construction of an energy level diagram as in Scheme 1.8.
The same type of effect reported for HBA1 was observed: raising of the energy level of the hemiketal and a more or less constant energy level of the quinoidal base as the acylated units increase. 5 However, no inversion of the energy levels of the hemiketal compared with quinoidal base was observed, suggesting that three acylated units are necessary. 6
The energy level diagram of the HBA1 was extended to the basic region; Scheme 1.10. Details can be found elsewhere (Mendoza et al. 2019).
After a pH jump to pH=5.5, for example, the system equilibrates between A, B, Cc, and Ct, but the most stable species is the quinoidal base A. In the case of a pH jump to the region of the mono‐anionic species, pH=8.5, the anionic quinoidal base becomes the most stable species.
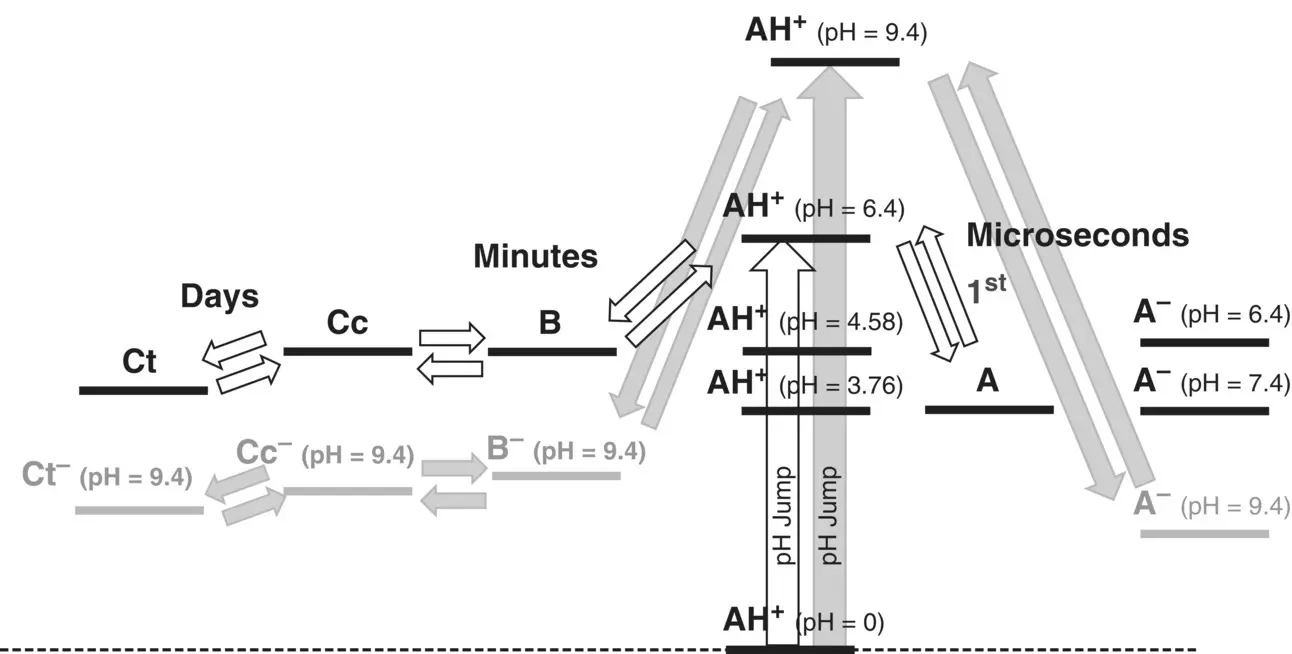
Scheme 1.10 Energy level diagram of the compound HBA 1extended to the mono‐anionic species. In the case of moderately acidic pHs the purple color of the quinoidal base is observed; at higher pH values it is the blue anionic quinoidal base that becomes more stable.
Source: Mendoza et al. 2018.
1.4.2 2‐Hydroxyflavylium Derivatives and Flavanones
The 2’‐hydroxyflavylium derivatives give rise to the formation of flavanones as shown in Scheme 1.11and Figure 1.6(Petrov et al. 2008; Slavcheva et al. 2018).
Figure 1.6shows the spectral variations after a direct pH jump to pH=8.1 (Slavcheva, et al. 2018). A few seconds after the pH jump the anionic hemiketal and cis ‐chalcone are formed ( Figure 1.6a). The system evolves and gives the anionic trans ‐chalcone after c. 40 minutes. However, equilibrium is reached four hours later, only after formation of the flavanone (FLV; Figure 1.6b), indicating that the flavanone is formed from the anionic trans ‐chalcone. Acidification of the flavanone does not give any spectral variation, but basification to pH=12.0 very quickly gives Ct 2‐( k obs= 3.3 s ‐1). When the titration of Ct 2‐by reverse pH jump is carried out, the absorption spectra of Ct ‐and Ctare observed according to the final pH. The spectrum of Ctis stable and the one of Ct ‐gives the flavanone as in Figure 1.6b.
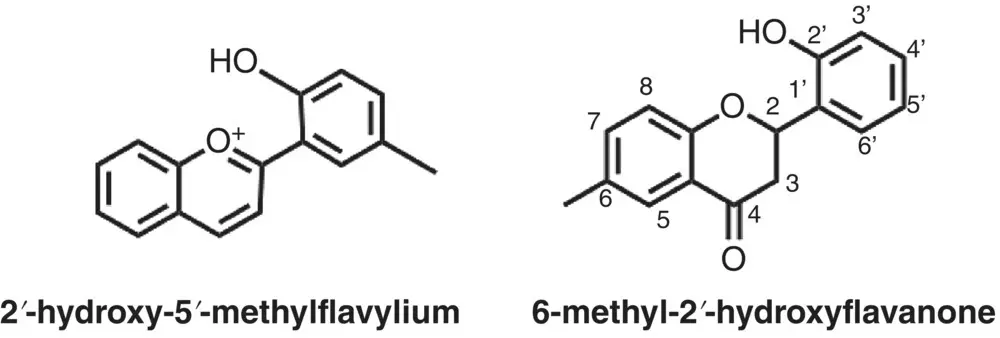
Scheme 1.11 2’‐hydroxy‐5’‐methylflavylium and its derived flavanone form.
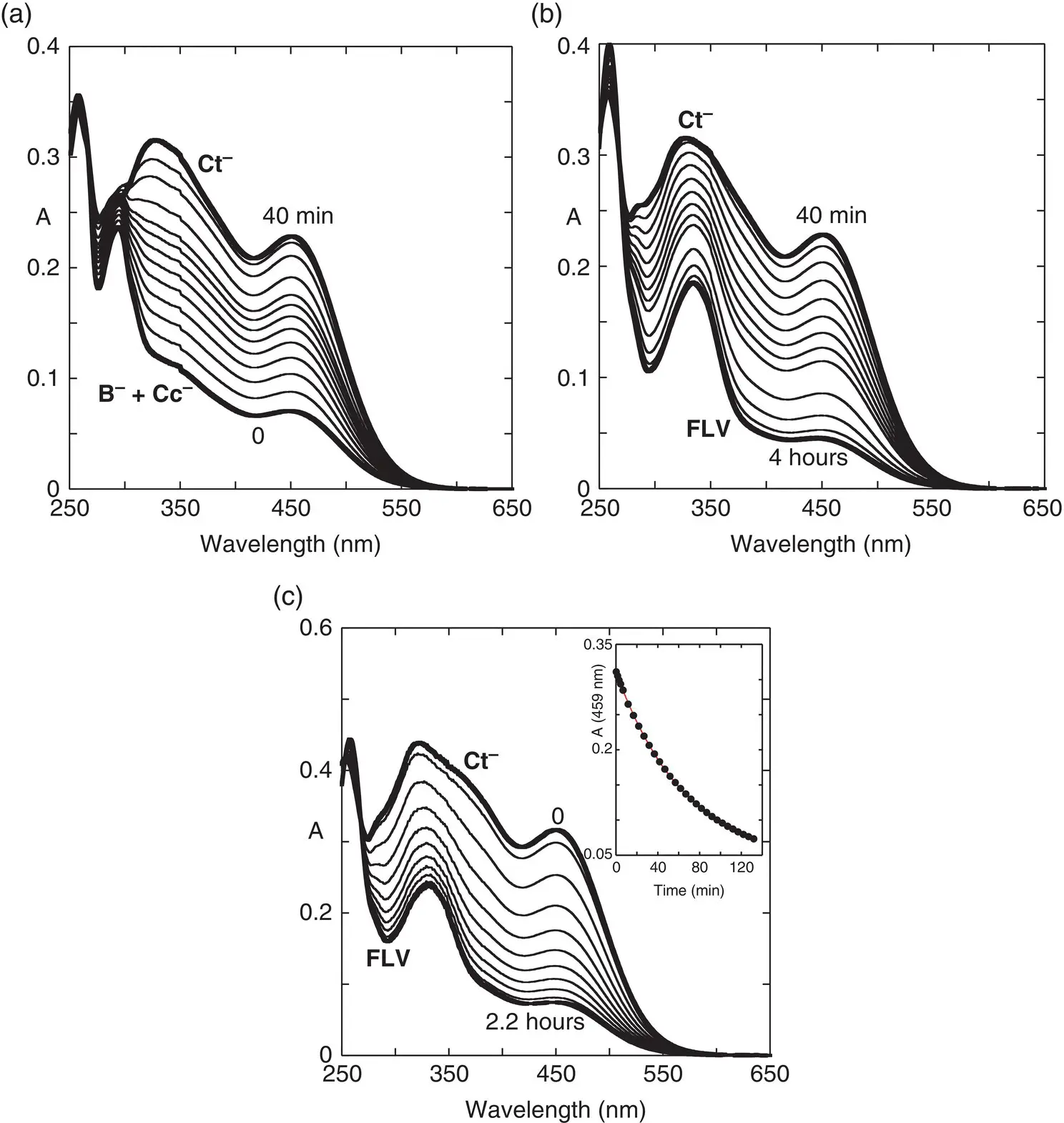
Figure 1.6 (a) Spectral variations after a direct pH jump from pH=1 to pH=8.1 of the compound 2’‐hydroxy‐5‐methylflavylium up to 40 minutes; (b) the same after 40 minutes up to 4 hours; (c) reverse pH jump from pH=12 to pH=8.4.
Source: Slavcheva, et al. 2018. © 2018 Elsevier.
This peculiar behavior permits to illustrate the concept of a timer at the molecular level with reset capacity ( Scheme 1.12).
The initial species is Ctat pH=4.0. The activation of the timer is made by changing the pH to pH=8.0, which allows the flavanone formation to start. To stop the timer the pH is reversed back to pH=4.0. The unreacted Ct ‐immediately gives the stable Ctand the flavanone does not react. The flavanone/ trans ‐chalcone molar ratio gives the extension of the reaction and the elapsed time. 7 To reset the timer two steps are needed: (i) the unlock step should be achieved by changing the pH to 12; the unreacted Ctand the flavanone (more precisely the anionic flavanone) give Ct 2‐, and (ii) after the end of the first step, the pH is shifted to 4.0 and the reaction goes back to Ct.
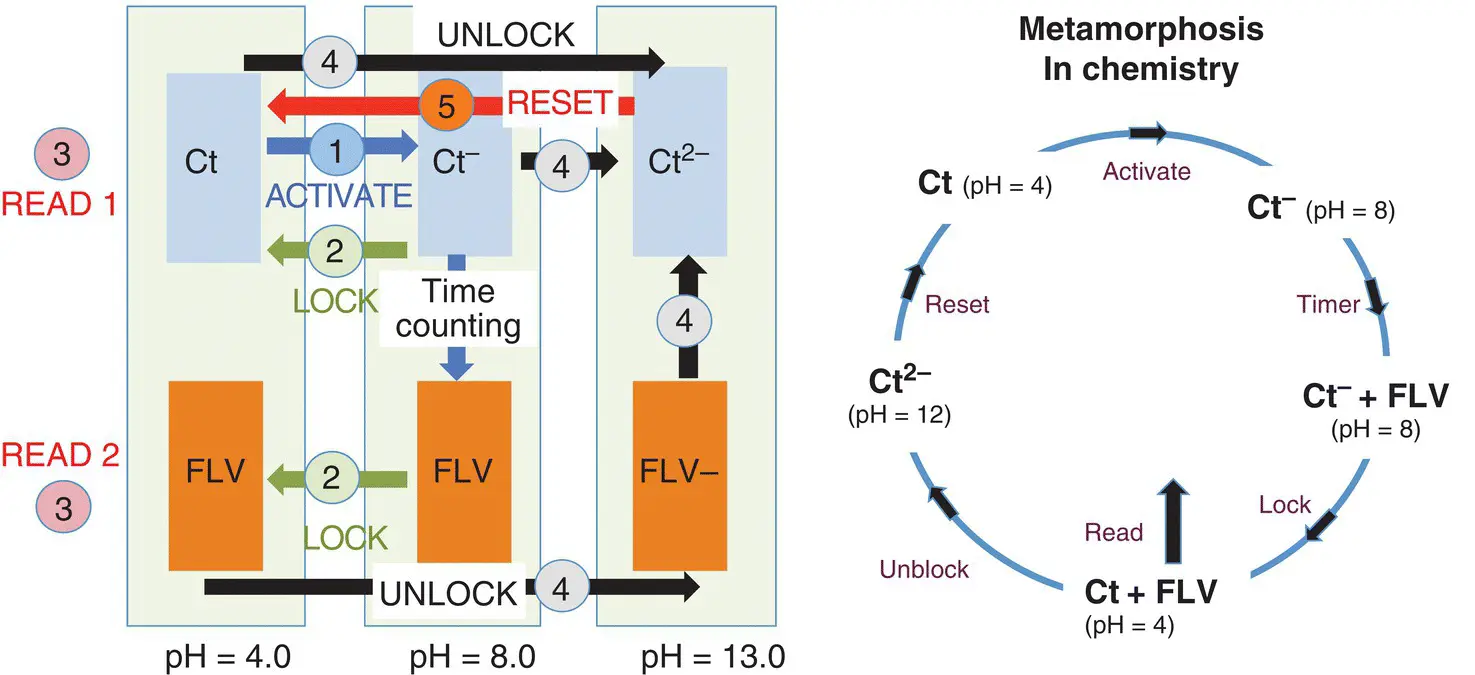
Scheme 1.12 Illustrating the concept of a timer with reset capacity through a metamorphosis cycle.
Source: Slavcheva, et al. 2018. © 2018 Elsevier.
1.4.3 6,8 Rearrangements
In Scheme 1.13, the 6,8 rearrangement is shown. Two necessary requirements are needed to observe this reaction in flavylium‐derived systems: (i) a hydroxyl group in position C5 and (ii) the lack of symmetry through the binary axis identified in Scheme 1.13by the traced line. For example, with the natural symmetrical 3‐deoxyanthocyanidins such as luteolinidin and apigeninidin, the 6,8 rearrangement is not observed. The 6,8 rearrangement in the flavylium cation was first reported (Jurd 1963) for 4’,5,7,8‐tetrahydroxyflavylium and for 6/8‐ C ‐glycosyl‐3‐deoxyanthocyanidins (Bjorøy et al. 2009). More recently, a complete study extended to the other species of the multistate present in moderately acidic medium was reported ( Scheme 1.13), R=Br (Cruz et al. 2016, 2017).
In very acidic medium, equilibrium is established between c. 50% of each flavylium cation. The two flavylium cation isomers were separated by HPLC and lyophilized. The usual pH jumps followed by stopped flow permitted us to obtain the absorption spectra of the two neutral quinoidal base isomers and study the respective kinetic processes. At pH=3.7 ( Figure 1.7), the equilibrium absorption spectrum can be fitted with the contributions of 0.45 A 6+ 0.45 A 8+ 0.1 Ct.
Читать дальшеИнтервал:
Закладка:
Похожие книги на «Recent Advances in Polyphenol Research»
Представляем Вашему вниманию похожие книги на «Recent Advances in Polyphenol Research» списком для выбора. Мы отобрали схожую по названию и смыслу литературу в надежде предоставить читателям больше вариантов отыскать новые, интересные, ещё непрочитанные произведения.
Обсуждение, отзывы о книге «Recent Advances in Polyphenol Research» и просто собственные мнения читателей. Оставьте ваши комментарии, напишите, что Вы думаете о произведении, его смысле или главных героях. Укажите что конкретно понравилось, а что нет, и почему Вы так считаете.












